Abstract
Superantigens (SAg) are a family of bacterial and viral proteins with strong immunostimulatory properties. SAg bound to major histocompatibility complex (MHC) class II molecules activate a high frequency of T cells and represent the most potent known activators of T cells to date. To explore the use of SAg for T cell-based tumor therapy we have created a tumor-reactive SAg by engineering a fusion protein composed of a tumor-reactive mAb (C215Fab) and the bacterial SAg staphylococcal enterotoxin A (SEA). A point mutation D227A was introduced at the major MHC class II binding site in SEA to reduce systemic toxicity. Treatment of tumor bearing mice with the Fab–SEA D227A fusion protein resulted in profound antitumor effects with a markedly reduced toxicity as compared with the wild-type Fab–SEA fusion protein. The reduced toxicity was probably due to a weak distribution of the SEA D227A fusion protein in tissues with a high MHC class II expression and low systemic cytokine levels as exhibited in mice and rabbits. The data presented demonstrate the efficacy of immunoconjugates containing a mutated SAg in directing a T cell attack against tumor cells with minimal systemic immune activation.
Tumor reactive cytotoxic T lymphocytes represent a potent effector mechanism against malignant cells presenting specific peptide antigens in the context of major histocompatibility complex (MHC) class I molecules (1). However, the frequency of these antigen-specific cytotoxic T lymphocytes is generally too low to interfere with the expansion of a progressively growing tumor. Superantigens (SAg) are a family of bacterial and viral proteins that bind to MHC class II molecules as unprocessed proteins and activate a large number of T cells bearing particular T cell receptor variable β (TCR Vβ) chains (2, 3). SAg induce strong cytokine production and cytotoxicity in CD4+ and CD8+ T lymphocytes. To explore the use of SAg in T cell-based tumor therapy, we have created a tumor reactive SAg by genetic fusion of the Fab part of a tumor-reactive mAb and the bacterial SAg staphylococcal enterotoxin A (SEA).
Strong antitumor responses are elicited by Fab–SEA fusion proteins in different experimental models (4–8). The mAb-targeted SAg directs an immune attack to the tumor which involves the induction of tumor infiltrating lymphocytes, local release of tumor suppressive cytokines, and the induction of apoptosis in the tumor cells (7–9). However, the Fab–SEA fusion proteins retain a substantial affinity for MHC class II molecules that results in the accumulation in MHC class II positive tissues followed by marked systemic T cell activation and accumulation of inflammatory cytokines in serum. It has also been shown that injection of high amounts of SAg induces a cytokine-dependent toxic shock syndrome (10).
The structure of the bacterial SAg SEB complexed with the human MHC class II molecule HLA-DR was recently resolved and demonstrated binding of the N-terminal domain of SEB to the HLA-DR α-chain (11). Site-directed mutagenesis of SEA in combination with the crystal structure of SEA confirmed the presence of an SEB-like low affinity MHC class II binding site and also demonstrated the existence of a Zn2+-dependent MHC class II binding site with moderate affinity in the C-terminal domain of the SEA molecule (12, 13).
To reduce the systemic immune activation elicited by the Fab–SEA fusion proteins we introduced a D227A point mutation in the C-terminal, MHC class II binding site of SEA. In vivo administration of the Fab–SEA D227A fusion protein at high doses demonstrated a strong antitumor response in the absence of excessive systemic immune activation and toxicity. The data demonstrate that highly toxic bacterially derived SAg may be genetically tailored into a synthetic tumor-specific SAg that is effective for cancer immunotherapy.
MATERIALS AND METHODS
Reagents.
Recombinant C215Fab–SEA (Fab–SEA) and C215Fab–SEA D227A (Fab–SEA D227A) were obtained by expression in Escherichia coli and purification to homogeneity as described (6).
Cloning, Expression, and Purification of C215Fab–SEA Fusion Protein.
The construction and expression of C215Fab–SEA was performed as described (6). The D227A mutation was introduced into the SEA gene by PCR-assisted mutagenesis as described (12). The fusion proteins were expressed in E. coli K-12 UL635 and purified on a protein G Sepharose column followed by a Mono S HR 5/5 column (Pharmacia LKB), and then the fractions containing C215Fab–SEA proteins were passed through a PD-10 column (Pharmacia LKB). The protein was >95% pure as determined by SDS/PAGE analysis.
In Vivo Tumor Model.
For induction of lung metastases, 1.5 × 105 B16 melanoma cells transfected with the C215 human tumor antigen (6) were injected i.v. into the tail vein of TCR Vβ3 transgenic (TG) mice (14). Animals were treated with three daily i.v. injections of 0.2 ml Fab–SEA or Fab–SEA D227A in PBS or PBS alone starting 1 day after tumor inoculation. Mice were killed after 3 weeks, and the frequency of lung metastases was counted. Tumor reduction was calculated as follows: 100 × (1 − number of metastases in Fab–SEA-treated mice/number of metastases in PBS-treated mice). For induction of lymph node tumors mice were anesthesized, the skin under the left fore limb was incized, and 2000 B16-C215 cells in 0.5 μl PBS were carefully injected into the left brachial lymph node using a sterile glass capillary tube and a high precision pump. The cut was sealed using tissue glue, and mice were checked for tumor growth after 5 days. Approximately 50% of the inoculated mice developed lymph node B16-C215 tumors and were selected for treatment with three daily injections of PBS, Fab–SEA (0.5 μg), or Fab–SEA D227A (50 μg) as described above. Mice were killed after 3 weeks, and the lymph node weights were determined.
Tissue Distribution and Toxicity of Fab–SEA Fusion Proteins.
New Zealand White male rabbits (Lidköpings Kaninfarm, Hasslösa, Sweden) were administered with 5.0 μg/kg (50 μCi/kg; 1 Ci = 37 GBq) of either 125I-labeled Fab–SEA or Fab–SEA D227A i.v. At 3 h postadministration, the animals were killed, and blood, the lungs, spleen, bone marrow, and skeletal muscle were taken and weighed. Plasma was prepared from the blood, and the radioactivity in the plasma and tissue samples was determined by γ-counting. Toxicity was determined after various doses of Fab–SEA proteins using New Zealand White male rabbits anesthesized with a mixture of isoflurane, O2, and N2O. Blood pressure was registered in cannulated femoral arteries for 3 min, and when stable values were obtained the test substance was injected i.v. at 1.0 ml/min and changes in blood pressure were registered. Moribund behavior such as severely depressed reflexes and breathing was used as estimation of toxicity in TCR Vβ3 TG mice.
Immunohistochemical Staining.
To detect cellular infiltration, Fab–SEA treatment was initiated 18 days after tumor challenge when large lung metastases were present. Staining for tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ) was performed 2 and 4 h, respectively, after the third injection of Fab–SEA fusion protein. Cryosections (8 μm) were fixed in 2% formaldehyde for 10 min, after blocking in avidin and biotin (Vector Laboratories blocking kit) sections were incubated with primary antibodies, anti-TNF-α (clone MP6-XT22, rat IgG1; PharMingen) or anti-IFN-γ (clone XMG 1.2, rat IgG1; PharMingen), or isotype matched controls in the presence of saponin. Sections were incubated with primary antibodies overnight and a biotinylated goat anti-rat IgG (Jackson Immuno Research) as described (7, 8).
Analysis of Serum Cytokine Levels.
Mice were i.v. injected with Fab–SEA fusion proteins and blood samples were drawn by caval vein puncture at various time points. All groups contained pooled sera from three animals; protein levels for the different cytokines were measured using standard ELISA methodology as described (14).
RESULTS
Fab–SEA D227A Elicits Strong Antitumor Responses with Reduced Toxicity.
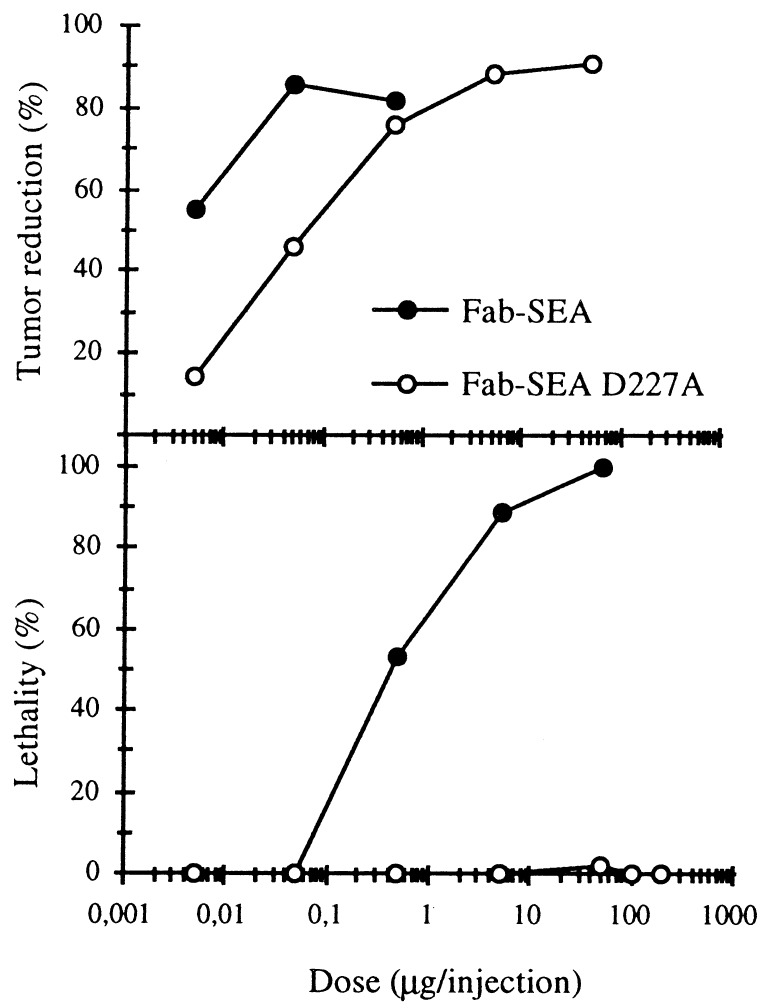
We have previously shown that TCR Vβ3 TG mouse strain represents a sensitive in vivo model for studying SEA-induced immunopharmacology (14). Following induction of B16-C215, pulmonary metastases in TCR Vβ3 TG mice there was a 90% reduction in the number of B16-C215 lung metastases after treatment with 0.05–0.5 μg of C215Fab–SEA (Fab–SEA), similar antitumor responses were seen using 5–50 μg C215Fab–SEA D227A (Fab–SEA D227A) (Fig. 1). A pronounced general toxicity was noted within the dose range of 5–50 μg of Fab–SEA with over 90% of the animals developing moribund behavior (15). In contrast, no negative side effects were recorded in mice treated with 5–200 μg of the Fab–SEA D227A (Fig. 1). Histopathological examination of affected animals demonstrated the presence of severe pulmonary oedema, whereas no negative side effects were observed in mice treated with 5–200 μg of the Fab–SEA D227A (data not shown).
Figure 1.
Tumor therapy and toxicity with C215Fab–SEA (Fab–SEA) and mutated C215Fab–SEA D227A (Fab–SEA D227A) in TCR Vβ3 TG mice. Reduction of pulmonary B16-C215 metastases are recorded after three injections of Fab–SEA proteins on a daily basis starting 1 day after tumor injection. Toxicity was noted after the third injection of Fab–SEA or Fab–SEA D227A in tumor-bearing TCR Vβ3 TG mice. Presented data represents the mean of three to four different experiments with six to eight animals in each experimental group.
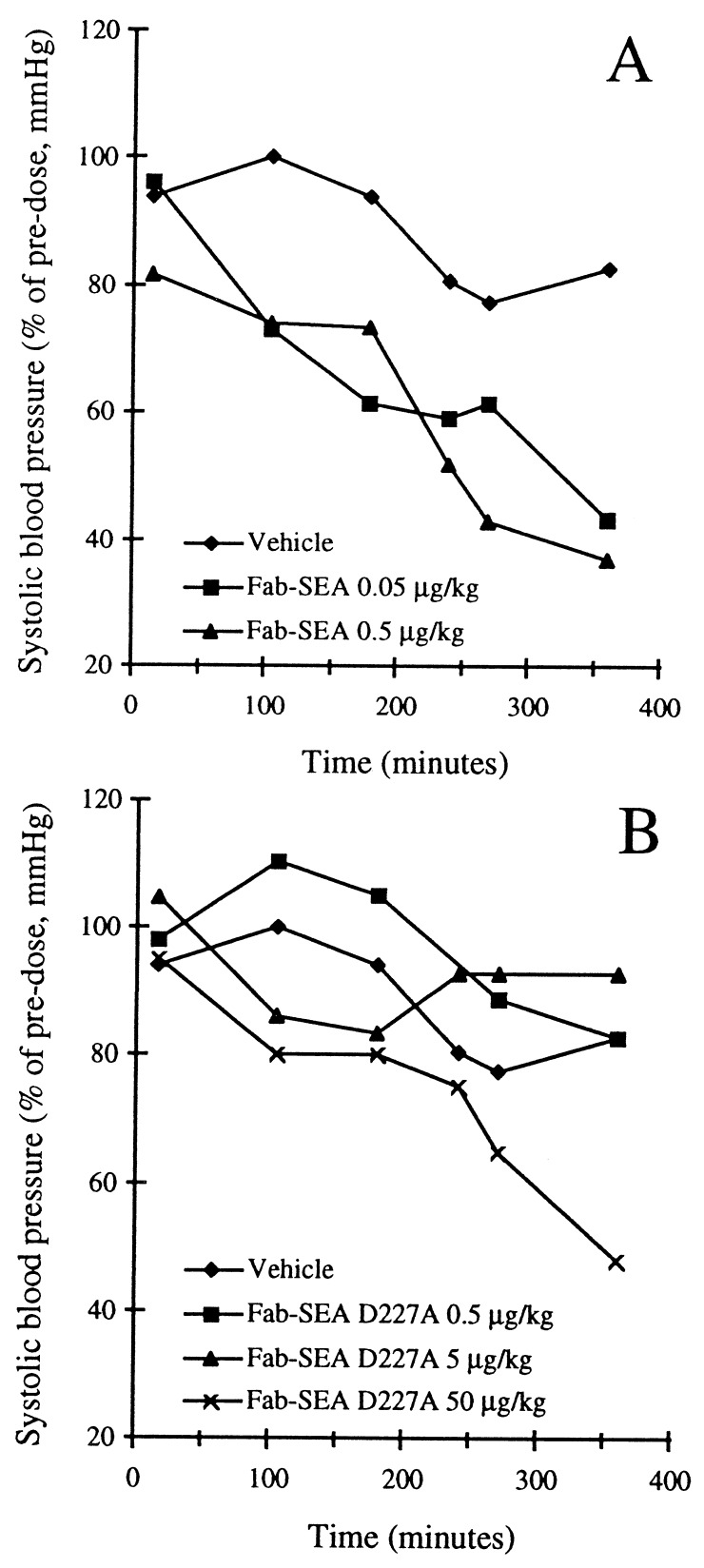
To evaluate toxicity of the Fab–SEA fusion proteins, cardiovascular parameters were monitored in anesthesized New Zealand White rabbits. Fab–SEA induced septic shock-like symptoms manifested as a pronounced drop in systolic blood pressure at 0.05–0.5 μg/kg (Fig. 2A) as well as a dose-dependent drop in white blood cell and platelet counts (data not shown). In contrast, more than 100-fold higher concentrations of Fab–SEA D227A were required to obtain similar responses (Fig. 2B).
Figure 2.
Drop in systolic blood pressure after injection of Fab–SEA (A) or Fab–SEA D227A (B) in anesthesized New Zealand White rabbits. The change in blood pressure is presented as percent of stable pre-dose pressure of each individual animal.
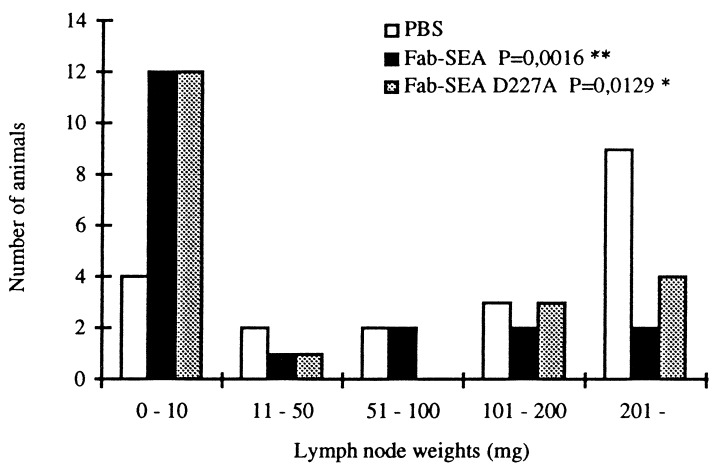
Minimal residual tumor disease in lymph nodes represents a major clinical limitation for the successful treatment of melanomas as well as a number of other major human tumor types including colorectal cancer, mammary tumors, and lung carcinoma. To analyze whether targeted SAg therapy would be efficient against established lymph node tumors, we microinjected B16-C215 melanoma cells into the brachial lymph node of TCR Vβ3 TG mice and initiated Fab–SEA therapy 5 days later. In untreated animals harboring lymph node tumors an ≈100-fold increase in lymph node tissue weight was recorded after 3 weeks due to the growth of the malignant tumor (Fig. 3). A significant reduction in lymph node tumor weight was recorded in response to treatment with either Fab–SEA or Fab–SEA D227A (Fig. 3). The therapeutic effect involved antibody targeting to the tumor area because no significant antitumor responses were recorded using C242Fab–SEA with irrelevant tumor specificity (data not shown).
Figure 3.
Tumor therapy of established lymph node B16-C215 tumors in TCR Vβ3 TG mice. Lymph nodes were microinjected with B16-C215 tumor cells and screened for tumor growth after 5 days. Mice with established tumor were selected for therapy. Accumulated data from two separate experiments with 19–20 animals in each group. Statistical significance of vehicle vs. fusion protein-treated animals was determined using the Mann–Whitney U test.
Tissue Distribution of the Fab–SEA Fusion Proteins.
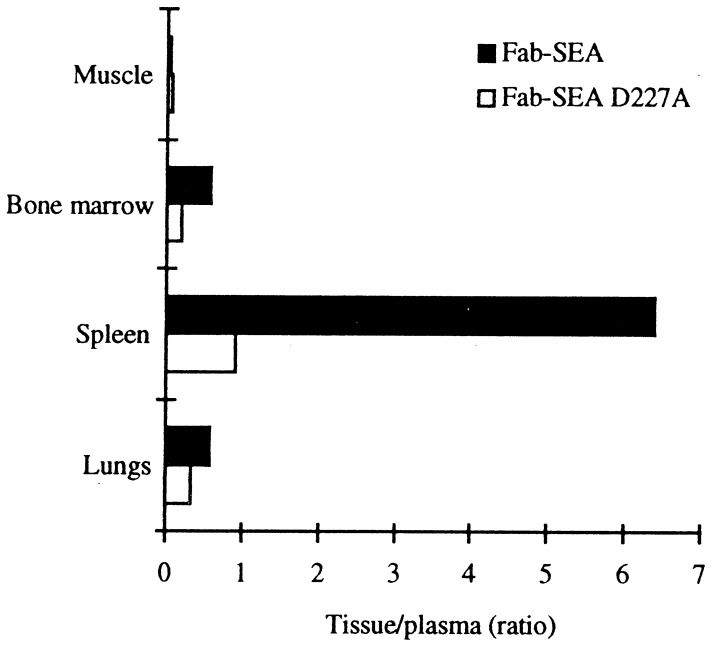
Fab–SEA D227A shows a significantly reduced affinity to MHC class II; to confirm that this mutant distributes differently from Fab–SEA in vivo the fusion proteins were 125I-labeled and their biodistribution monitored in New Zealand White rabbits. Fab–SEA and Fab–SEA D227A exhibited a poor distribution in skeletal muscle, whereas the tissue-to-plasma ratio for Fab–SEA was high in bone marrow, lung, and spleen (Fig. 4). The tissue-to-plasma ratio for Fab–SEA D227A in the spleen, which contains large numbers of cells expressing MHC class II, was reduced by 7-fold when compared with nonmutated Fab–SEA. This demonstrates that mutation of the MHC class II high-affinity domain in SEA significantly reduces distribution to tissues with high levels of MHC class II expression.
Figure 4.
Tissue distribution in New Zealand White rabbits after i.v. injection of of 125I-labeled Fab–SEA and 125I-labeled Fab–SEA D227A expressed as tissue-to-plasma ratio.
Localized Cytokine Production in Tumor-Bearing Animals Treated with Fab–SEA D227A.
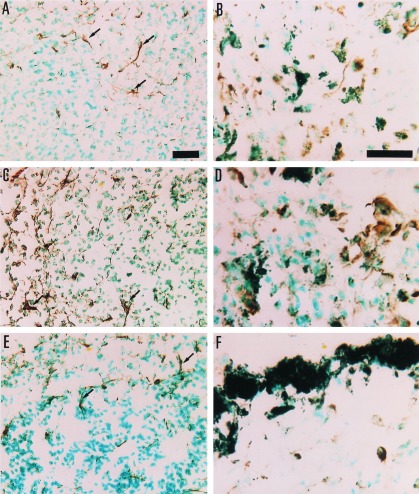
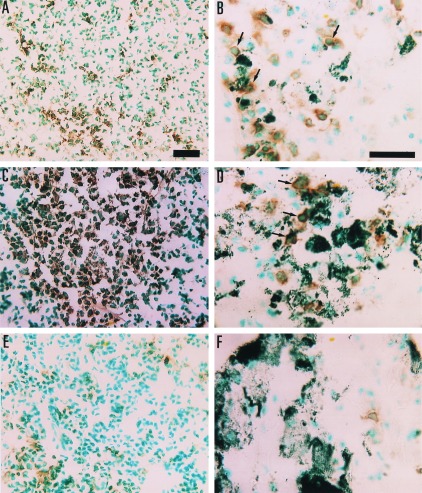
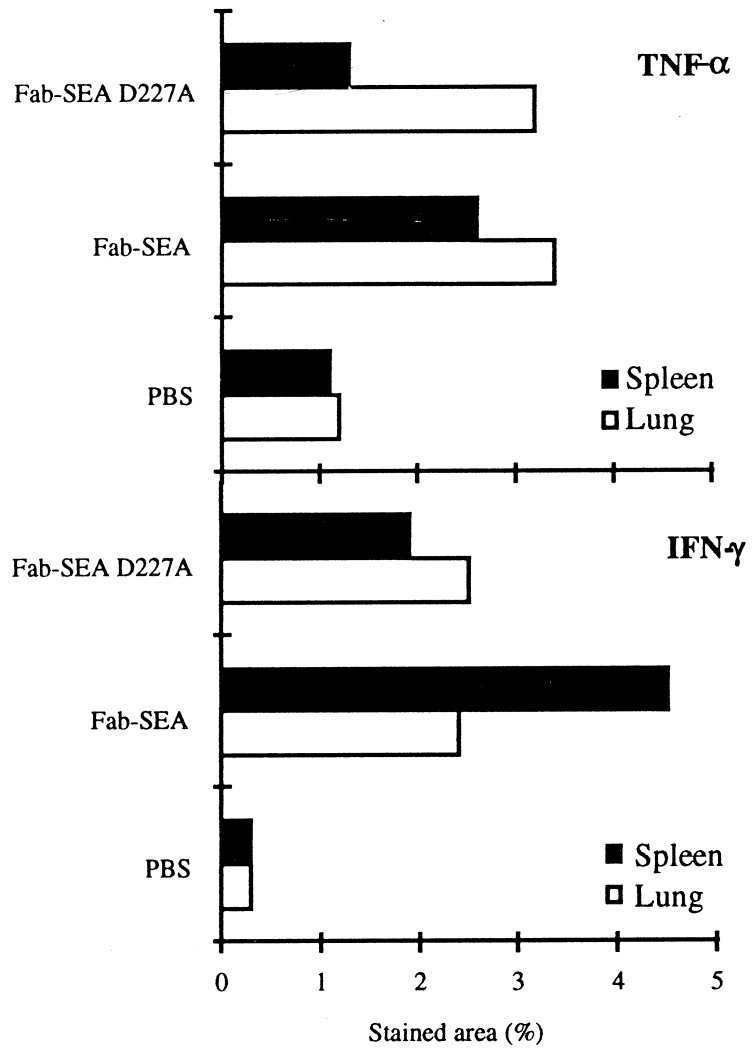
TCR Vβ3 TG mice carrying pulmonary metastases of the B16-C215 melanoma were treated with three injections of Fab–SEA (0.05 μg/injection) or Fab–SEA D227A (50 μg/injection) on a daily basis. Immunohistochemistry was performed on spleen and lung to determine the T cell infiltration and production of TNF-α and IFN-γ. Induction of tumor-infiltrating lymphocytes and a strong production of TNF-α and IFN-γ was observed in the tumor area following treatment with Fab–SEA or Fab–SEA D227A (Figs. 5 and 6). PBS-treated animals contained no tumor-infiltrating lymphocytes and only marginal amounts of TNF-α or IFN-γ production were seen in the lung and spleen (Figs. 5 and 6). Substantial production of TNF-α and IFN-γ was detected in the spleen after treatment with Fab–SEA (Figs. 5 and 6). However, a dramatic reduction in the number of cytokine-producing spleen cells was recorded after treatment with Fab–SEA D227A (Figs. 5 and 6). Image analysis confirmed the presence of similar numbers of TNF-α and IFN-γ positive cells in the lung after treatment with Fab–SEA wild type or the Fab–SEA D227A (Fig. 7). The ratio between TNF-α- and IFN-γ-stained cells in the lung versus the spleen increased 2- to 3-fold after therapy with the mutant protein Fab–SEA D227A (50 μg/injection) when compared with Fab–SEA wild type (0.05 μg/injection) (Fig. 7).
Figure 5.
Micrographs of TNF-α positive cells in histological sections from spleen (A, C, and E) and lung (B, D, and F) of B16-C215 tumor-bearing TCR Vβ3 TG mice. The immunoreaction is visualized by diaminobenzidine, giving a brown color at the site of reaction. A and B are from mice treated with the Fab–SEA D227A mutant, C and D are from mice treated with the Fab–SEA wild type, and E and F are from a control animal injected with PBS. In treated animals (B and D) TNF-α positive cells are seen dispersed among melanin-pigmented B16-C215 melanoma cells in the lung. TNF-α staining associated with dendritic-like cells are indicated by arrows. (Bars = 25 μm for A–F.)
Figure 6.
Micrographs of IFN-γ positive cells in histological sections from spleen (A, C, and E) and lung (B, D, and F) of B16-C215 tumor-bearing TCR Vβ3 TG mice. The immunoreaction is visualized by diaminobenzidine, giving a brown color at the site of reaction. A and B are from mice treated with Fab–SEA D227A, C and D are from mice treated with Fab–SEA, and E and F are from a control animal injected with PBS. Note the close proximity of IFN-γ positive cells and melanin-pigmented B16-C215 cells in treated animals in B and D (arrows). (Bars = 25 μm for A–F.)
Figure 7.
Quantitation of cells stained for TNF-α and IFN-γ in histological section from spleen and lung of B16-C215 tumor-bearing TCR Vβ3 TG mice. Quantitation of stained cells was performed by image analysis and data represents mean of the positively stained area after serial scanning of whole cryostat sections.
Low Levels of Systemic Cytokines After Treatment with Fab–SEA D227A.
To further quantitate the systemic immune response by Fab–SEA and Fab–SEA D227A, we analyzed the serum levels of a panel of cytokines in TCR Vβ3 TG mice following injection with equipotent, therapeutic doses of Fab–SEA and Fab–SEA D227A (0.05 μg and 50 μg, respectively). Repeated injections of Fab–SEA resulted in a strong production of interleukin (IL) 2, IFN-γ, TNF-α, and IL-6 (Fig. 8). As expected, a reduction in the systemic levels of the tested cytokines was seen when comparing the effects of Fab–SEA with the Fab–SEA D227A (Fig. 8). A 10- to 100-fold reduction in the serum levels of IL-2 and IFN-γ and a 100- to 1000-fold reduced amount of TNF-α and IL-6 was recorded in animals treated with Fab–SEA D227A (Fig. 8).
Figure 8.
Serum cytokine levels after treatment with wild-type and mutated C215Fab–SEA fusion proteins in TCR Vβ3 TG mice. Mice were injected one, two, or three times (1×, 2×, and 3×, respectively) with Fab–SEA and Fab–SEA D227A and serum levels of IL-2, TNF-α, and IL-6 were analyzed after 2 h and IFN-γ after 4 h. Serum levels of IL-2, TNF-α, and IFN-γ are in units/ml, and IL-6 levels are in pg/ml.
DISCUSSION
Antibody-targeted SAg express certain properties that are favorable for T cell-based tumor immunotherapy. These are as follows: (i) the Fab–SAg protein is highly potent and activates about 10% of T lymphocytes in humans and certain mouse strains, (ii) Fab–SAg proteins efficiently target T cells against antigen positive tumor cells in the absence of MHC class II molecules, and (iii) as opposed to other mAb based T cell therapies, such as bifunctional antibodies, the Fab–SAg proteins do not interact with T cells in a soluble form, but require the presentation on a cell surface. Indeed, it has been shown that tumor-targeted SAg induce strong antitumor responses in experimental tumor models using normal C57BL/6 mice, which seems to reflect a significant induction of tumor infiltrating T lymphocytes (6–8, 16).
Bacterial SAg including SEA have evolved under the evolutionary pressure of the mammalian immune system. It has been argued that the strong MHC class II binding characteristic of bacterial SAg in combination with the potent T cell activation facilitates bacterial invasion by the eradication of MHC II+ antigen-presenting cells, resulting in a perturbed specific immunity (17). Furthermore, the potent immunostimulatory properties of these proteins induces high systemic levels of a large panel of inflammatory cytokines that may lead to a toxic shock syndrome (14, 18–20).
Mice are significantly less sensitive to toxic shock induced by bacterial SAg when compared with primates and rabbits and are not useful for predicting toxicity in humans. This discrepancy may be explained by the poor affinity of the bacterial SAg to murine MHC class II. Indeed, it has been shown that mice transgenic for human MHC class II are supersensitive to septic shock and produce high levels of TNF-α after challenge with bacterial SAg (21). Also, mice transgenic for the SEA-reactive TCR Vβ3 chain produce high levels of inflammatory cytokines and may develop severe septicemia following exposure to SEA or SEA-secreting staphylococci (14, 22). In these mice, 10-fold lower concentrations of Fab–SEA fusion protein are required compared with normal C57BL/6 mice for significant antitumor responses that are due to a larger fraction of responding T cells.
To reduce the systemic immune activation by Fab–SAg we introduced a point mutation at the high-affinity MHC class II binding site in the SEA moiety of the Fab–SEA fusion protein. The mutant Fab–SEA D227A protein displayed a 1000-fold reduction in MHC class II binding but retained the ability to target cytotoxic T lymphocytes in a MHC class II independent manner (12).
Successful treatment of TCR Vβ3 TG mice carrying B16-C215 melanoma cells using the Fab–SEA D227A protein was seen at doses which induced only marginal systemic immune activation and toxicity, and this was mirrored in rabbits where high doses of Fab–SEA D227A could be administered without any signs of toxic shock. In contrast, wild-type Fab–SEA induced a pronounced systemic cytokine release at therapeutically active doses in mice and pronounced toxicity in rabbits at >100-fold lower concentrations than Fab–SEA D227A. The evaluation of toxicity in mice and rabbits corroborates previous preliminary experiments in TCR Vβ3 TG mice, which indicated that the therapeutic window was greatly improved using the mutated Fab–SEA D227A compared with treatment with the Fab–SEA protein (15).
Notably, a 100- to 1000-fold reduction in serum levels of IL-6 and TNF-α was seen in mice after treatment with Fab–SEA D227A when compared with the wild-type protein, although the mutated protein was given at 1000-fold higher concentrations. Increased systemic levels of IL-6 and TNF-α have been reported to correlate with the severity of toxic shock in vivo (20, 23, 24). It is therefore reasonable to assume that the reduction in serum IL-6 and TNF-α is related to the improved therapeutic window seen in Fab–SEA D227A-treated animals. It was recently shown that bivalent binding of SEA to MHC class II molecules induced expression of TNF and IL-1 whereas monovalent binding of SEA D227A failed to induce the expression of these cytokines (25). The low serum levels of IL-6 and TNF-α observed in animals treated with Fab–SEA D227A suggests that the interference with MHC class II binding and cross-linking on macrophages may contribute to the reduced IL-6 and TNF-α expression in vivo.
Immunohistochemical analysis of spleens taken from mice treated with Fab–SEA D227A showed a significant reduction in the number of TNF-α and IFN-γ producing cells compared with Fab–SEA-treated mice. Importantly, production of TNF-α and IFN-γ was still induced locally in the tumor area by the Fab–SEA D227A fusion protein. The growth of murine B16 melanoma cells is sensitive to IFN-γ and TNF and preliminary mAb-neutralization experiments in vivo suggest that IFN-γ is required for successful eradication of the tumor (unpublished observation).
In this report we describe the properties of a synthetic Fab–SAg fusion protein in which the MHC class II reactivity has been substituted with tumor specificity, allowing cytokine producing T lymphocytes to be specifically targeted to the tumor area. Thus, the harmful bacterial SAg exotoxins can be converted into tolerable immunotoxins which are applicable in therapy of malignant diseases.
Acknowledgments
We thank Ms. M. Celander, Ms. K. Behm, and Ms. C. Valfridsson for skillful technical assistance; Drs. G. Forsberg and L. Abrahmsén for the production of fusion proteins; and Dr. K. Dawe for critical reading of the manuscript.
ABBREVIATIONS
- SEA
staphylococcal enterotoxin A
- SAg
superantigens
- MHC
major histocompatibility complex
- IFN
interferon
- TNF
tumor necrosis factor
- TCR Vβ
T cell receptor variable β
- TG
transgenic
- IL
interleukin
References
- 1.Urban J L, Schreiber H. Annu Rev Immunol. 1992;10:617–644. doi: 10.1146/annurev.iy.10.040192.003153. [DOI] [PubMed] [Google Scholar]
- 2.Marrack P, Kappler J. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 3.Fraser J D. Nature (London) 1989;339:221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- 4.Dohlsten M, Hedlund G, Akerblom E, Lando P A, Kalland T. Proc Natl Acad Sci USA. 1991;88:9287–9291. doi: 10.1073/pnas.88.20.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lando P A, Dohlsten M, Hedlund G, Brodin T, Sansom D, Kalland T. Immunology. 1993;80:236–241. [PMC free article] [PubMed] [Google Scholar]
- 6.Dohlsten M, Abrahmsén L, Björk P, Lando P A, Hedlund G, Forsberg G, Brodin T, Gascoigne N R, Forberg C, Lind P, Kalland T. Proc Natl Acad Sci USA. 1994;91:8945–8949. doi: 10.1073/pnas.91.19.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dohlsten M, Hansson J, Ohlsson L, Litton M, Kalland T. Proc Natl Acad Sci USA. 1995;92:9791–9795. doi: 10.1073/pnas.92.21.9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohlsten M, Lando P A, Björk P, Abrahmsén L, Ohlsson L, Lind P, Kalland T. Cancer Immunol Immunother. 1995;41:162–168. doi: 10.1007/BF01521342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litton M, Dohlsten M, Lando P A, Kalland T, Ohlsson L, Andresson J, Andersson U. Eur J Immunol. 1996;26:1–9. doi: 10.1002/eji.1830260102. [DOI] [PubMed] [Google Scholar]
- 10.Miethke T, Wahl C, Regele D, Gaus H, Heeg K, Wagner H. Immunobiology. 1993;189:270–284. doi: 10.1016/S0171-2985(11)80362-1. [DOI] [PubMed] [Google Scholar]
- 11.Jardetzky T S, Brown J H, Gorga J C, Stern L J, Urban R G, Chi Y I, Stauffacher C, Strominger J L, Wiley D C. Nature (London) 1994;368:711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- 12.Abrahmsén L, Dohlsten M, Segren S, Björk P, Jonsson E, Kalland T. EMBO J. 1995;14:2978–2986. doi: 10.1002/j.1460-2075.1995.tb07300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schad E M, Zaitseva I, Zaitsev V N, Dohlsten M, Kalland T, Schlievert P M, Ohlendorf D H, Svensson L A. EMBO J. 1995;14:3292–3300. doi: 10.1002/j.1460-2075.1995.tb07336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dohlsten M, Bjorklund M, Sundstedt A, Hedlund G, Samson D, Kalland T. Immunology. 1993;79:520–527. [PMC free article] [PubMed] [Google Scholar]
- 15.Antonsson P, Hansson J, Kalland T, Lando P A, Ohlsson L, Schad E, Svensson A, Dohlsten M. Springer Semin Immunopathol. 1996;17:307–410. doi: 10.1007/BF01795137. [DOI] [PubMed] [Google Scholar]
- 16.Lando P A, Dohlsten M, Ohlsson L, Kalland T. Int J Cancer. 1995;62:466–471. doi: 10.1002/ijc.2910620418. [DOI] [PubMed] [Google Scholar]
- 17.Dohlsten M, Hedlund G, Kalland T. Immunol Today. 1991;12:147–150. doi: 10.1016/S0167-5699(05)80043-X. [DOI] [PubMed] [Google Scholar]
- 18.Sundstedt A, Dohlsten M, Hedlund G, Hoiden I, Bjorklund M, Kalland T. Immunology. 1994;82:117–125. [PMC free article] [PubMed] [Google Scholar]
- 19.Baschieri S, Lees R K, Lussow A R, MacDonald H R. Eur J Immunol. 1993;23:2661–2666. doi: 10.1002/eji.1830231041. [DOI] [PubMed] [Google Scholar]
- 20.Miethke T, Duschek K, Wahl C, Heeg K, Wagner H. Eur J Immunol. 1993;23:1494–1500. doi: 10.1002/eji.1830230715. [DOI] [PubMed] [Google Scholar]
- 21.Yeung R S M, Penninger J M, Kundig T, Khoo W, Ohashi P S, Kroemer G, Mak T W. Eur J Immunol. 1996;26:1074–1082. doi: 10.1002/eji.1830260518. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y X, Abdelnour A, Kalland T, Tarkowski A. Infect Immun. 1995;63:4463–4469. doi: 10.1128/iai.63.11.4463-4469.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Ann Surg. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehindate K, Thibodeau J, Dohlsten M, Kalland T, Sékaly R, Mourad W. J Exp Med. 1995;182:1573–1577. doi: 10.1084/jem.182.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]