Abstract
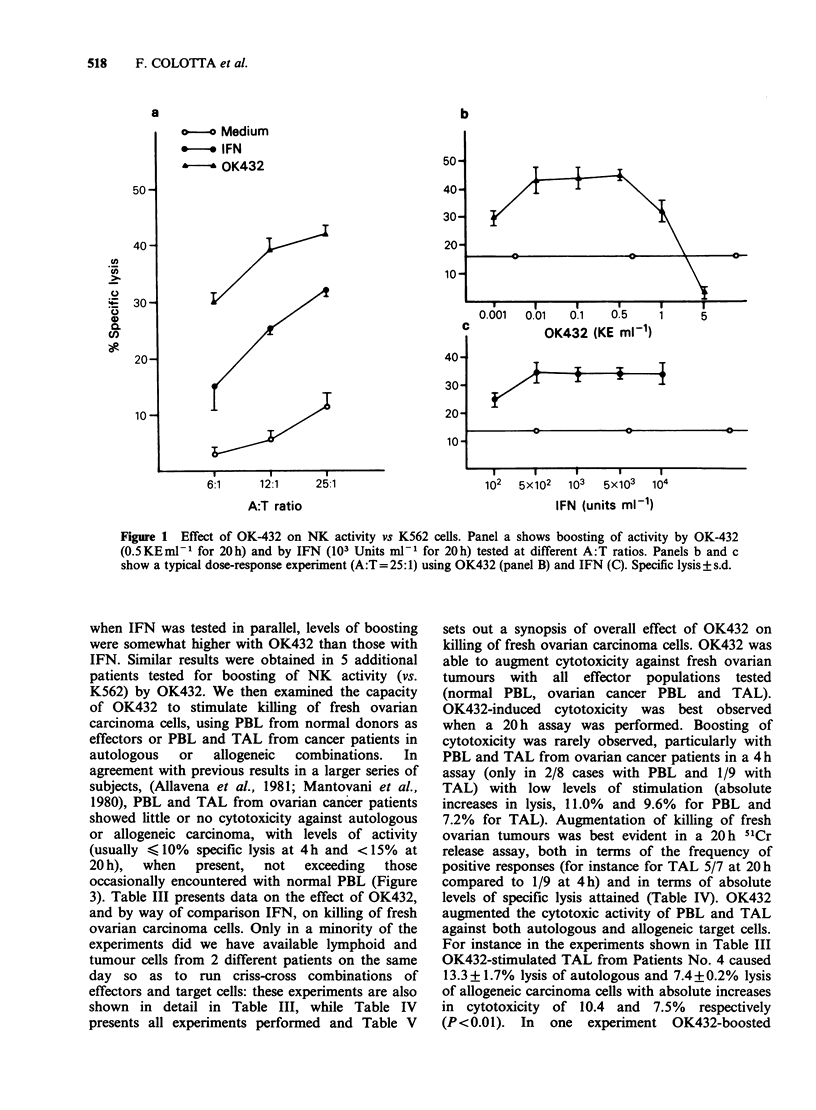
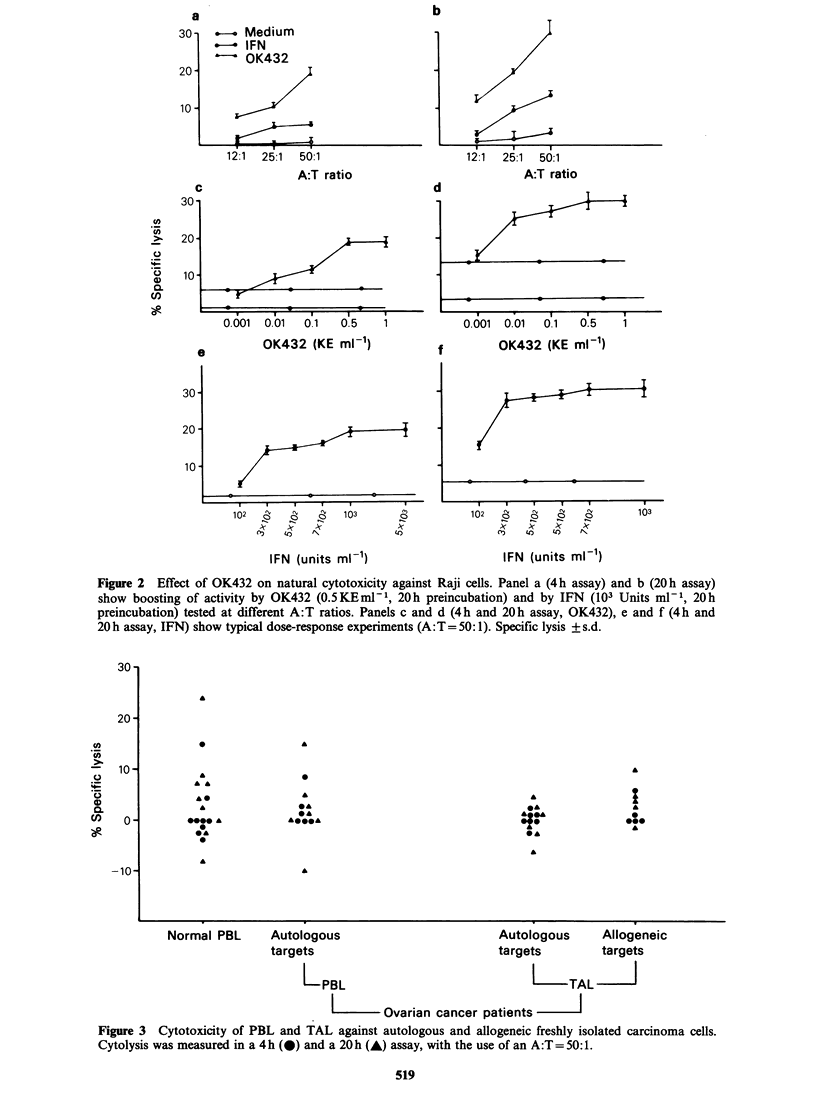
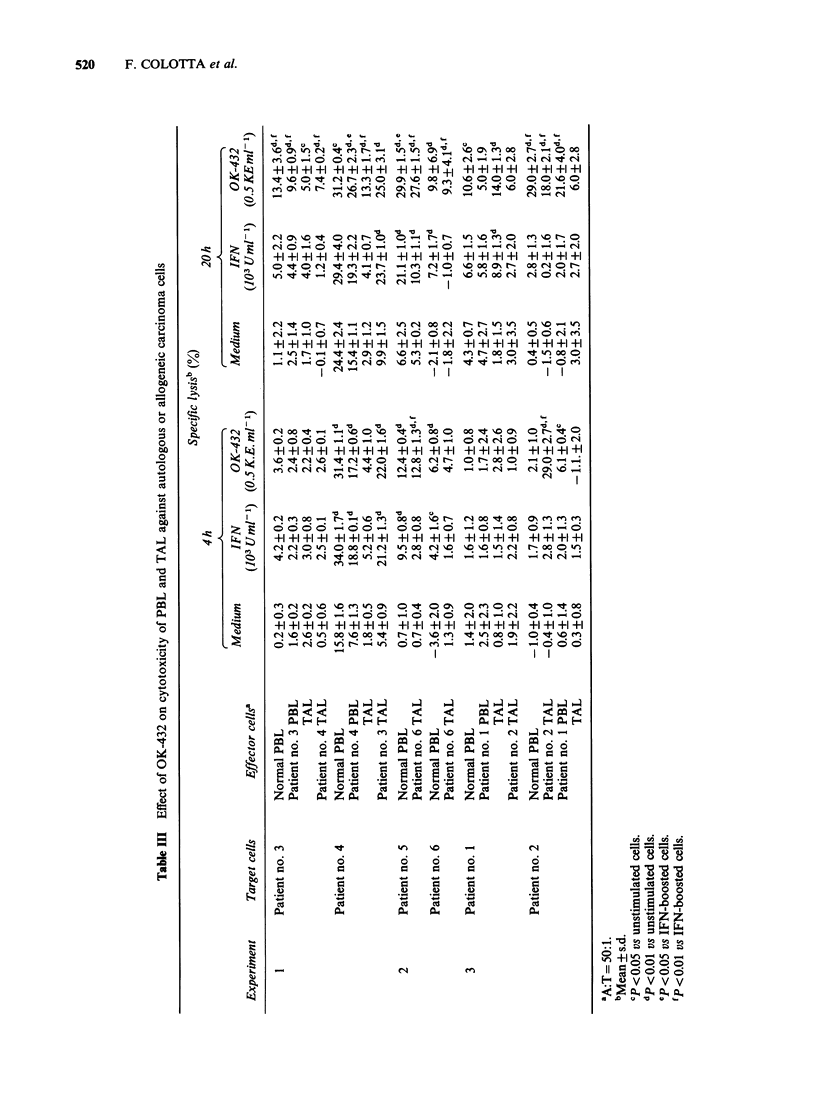
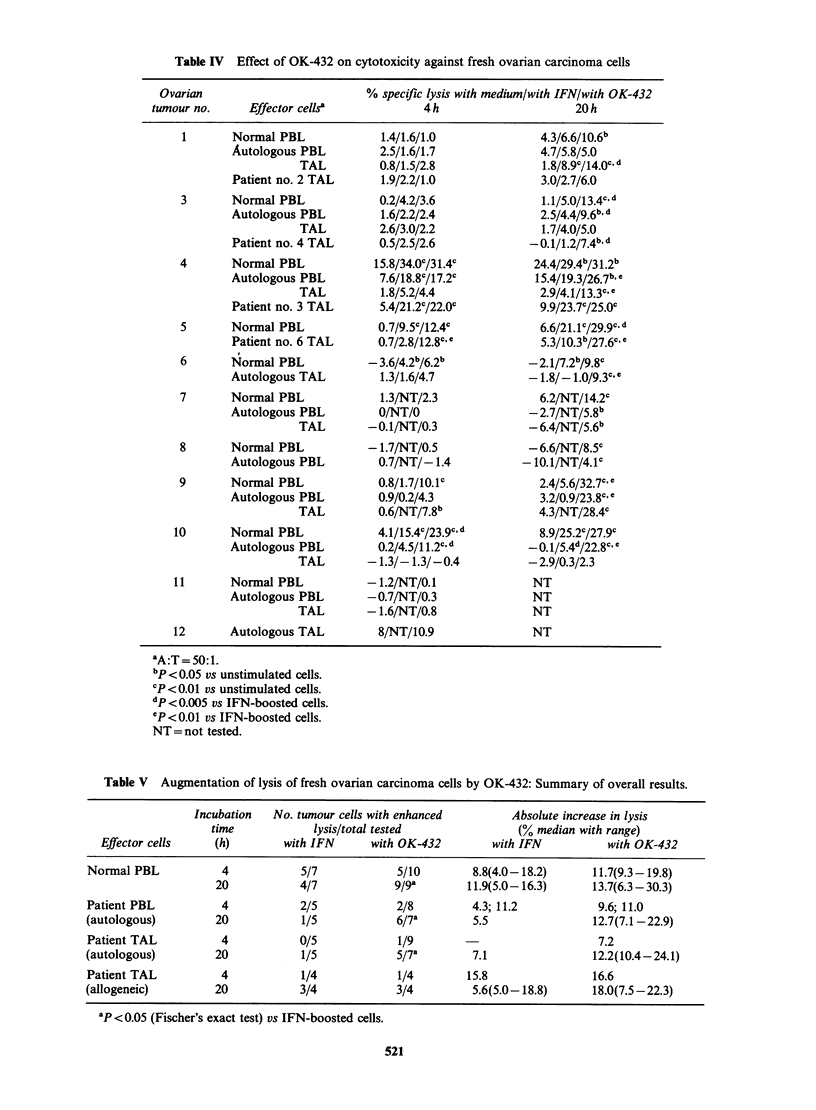
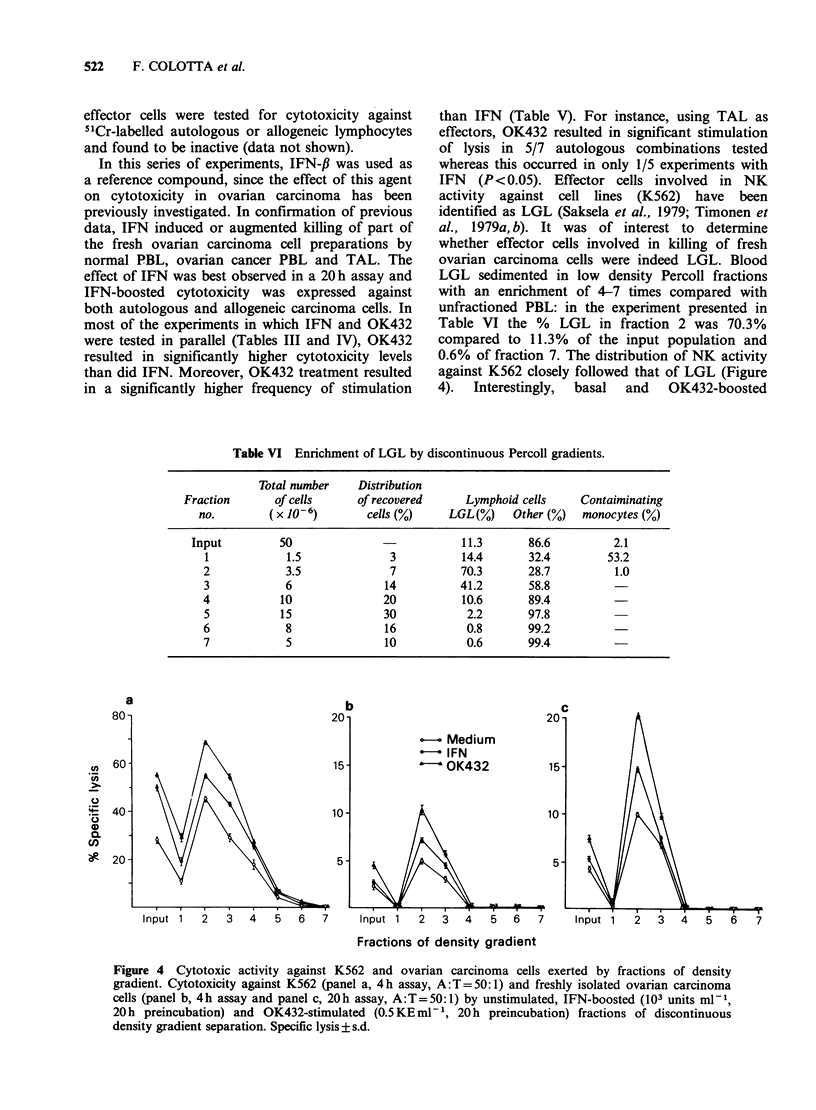
The streptococcal preparation OK432 was studied for its effects on natural killer (NK) activity of peripheral blood lymphocytes (PBL) from normal donors and from ovarian cancer patients, and of tumour-associated lymphocytes (TAL) from peritoneal effusions. OK432 augmented NK activity against the susceptible K562 line and induced killing of the relatively resistant Raji line. Freshly isolated ovarian carcinoma cells were relatively resistant to killing by unstimulated PBL and TAL. OK432 induced significant, though low, levels of cytotoxicity against 51Cr-labelled ovarian carcinoma cells. Augmentation of killing of fresh tumour cells by OK432 was best observed in a 20 h assay and both autologous and allogeneic targets were lysed. PBL were separated on discontinuous Percoll gradients. Unstimulated and OK432-boosted activity were enriched in the lower density fractions where large granular lymphocytes (LGL) and activity against K562 were found. Thus, OK432 augments NK activity of PBL and TAL in human ovarian carcinomas and induces low, but significant, levels of killing of fresh tumour cells. Effector cells involved in killing of fresh ovarian tumours copurify with LGL on discontinuous gradients of Percoll.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allavena P., Introna M., Mangioni C., Mantovani A. Inhibition of natural killer activity by tumor-associated lymphoid cells from ascites ovarian carcinomas. J Natl Cancer Inst. 1981 Aug;67(2):319–325. [PubMed] [Google Scholar]
- Allavena P., Introna M., Sessa C., Mangioni C., Mantovani A. Interferon effect on cytotoxicity of peripheral blood and tumor-associated lymphocytes against human ovarian carcinoma cells. J Natl Cancer Inst. 1982 Apr;68(4):555–562. [PubMed] [Google Scholar]
- Cudkowicz G., Hochman P. S. Do natural killer cells engage in regulated reactions against self to ensure homeostasis? Immunol Rev. 1979;44:13–41. doi: 10.1111/j.1600-065x.1979.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Introna M., Allavena P., Biondi A., Colombo N., Villa A., Mantovani A. Defective natural killer activity within human ovarian tumors: low numbers of morphologically defined effectors present in situ. J Natl Cancer Inst. 1983 Jan;70(1):21–26. [PubMed] [Google Scholar]
- Kedar E., Ikejiri B. L., Bonnard G. D., Herberman R. B. A rapid technique for isolation of viable tumor cells from solid tumors: use of the tumor cells for induction and measurement of cell-mediated cytotoxic responses. Eur J Cancer Clin Oncol. 1982 Oct;18(10):991–1000. doi: 10.1016/0277-5379(82)90248-6. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sessa C., Bolis G., Mangioni C. Natural killer activity of lymphoid cells isolated from human ascitic ovarian tumors. Int J Cancer. 1980 May 15;25(5):573–582. doi: 10.1002/ijc.2910250505. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Sessa C., Peri G., Allavena P., Introna M., Polentarutti N., Mangioni C. Intraperitoneal administration of Corynebacterium parvum in patients with ascitic ovarian tumors resistant to chemotherapy: effects on cytotoxicity of tumor-associated macrophages and NK cells. Int J Cancer. 1981;27(4):437–446. doi: 10.1002/ijc.2910270404. [DOI] [PubMed] [Google Scholar]
- Moore M., Taylor G. M., White W. J. Susceptibility of human leukaemias to cell-mediated cytotoxicity by interferon-treated allogeneic lymphocytes. Cancer Immunol Immunother. 1982;13(1):56–61. doi: 10.1007/BF00200202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshimi K., Kano S., Takaku F., Okumura K. Augmentation of mouse natural killer cell activity by a streptococcal preparation, OK-432. J Natl Cancer Inst. 1980 Dec;65(6):1265–1269. [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela E., Timonen T., Ranki A., Häyry P. Morphological and functional characterization of isolated effector cells responsible for human natural killer activity to fetal fibroblasts and to cultured cell line targets. Immunol Rev. 1979;44:71–123. doi: 10.1111/j.1600-065x.1979.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen T., Ranki A., Saksela E., Häyry P. Human natural cell-mediated cytotoxicity against fetal fibroblasts. III. Morphological and functional characterization of the effector cells. Cell Immunol. 1979 Nov;48(1):121–132. doi: 10.1016/0008-8749(79)90105-9. [DOI] [PubMed] [Google Scholar]
- Timonen T., Saksela E. Isolation of human NK cells by density gradient centrifugation. J Immunol Methods. 1980;36(3-4):285–291. doi: 10.1016/0022-1759(80)90133-7. [DOI] [PubMed] [Google Scholar]
- Timonen T., Saksela E., Ranki A., Häyry P. Fractionation, morphological and functional characterization of effector cells responsible for human natural killer activity against cell-line targets. Cell Immunol. 1979 Nov;48(1):133–148. doi: 10.1016/0008-8749(79)90106-0. [DOI] [PubMed] [Google Scholar]
- Uchida A., Hoshino T. Clinical studies on cell-mediated immunity in patients with malignant disease. I. Effect of immunotherapy with OK-432 on lymphocyte subpopulation and phytomitogen responsiveness in vitro. Cancer. 1980 Feb;45(3):476–483. doi: 10.1002/1097-0142(19800201)45:3<476::aid-cncr2820450311>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Uchida A., Hoshino T. Reduction of suppressor cells in cancer patients treated with OK-432 immunotherapy. Int J Cancer. 1980 Oct 15;26(4):401–404. doi: 10.1002/ijc.2910260403. [DOI] [PubMed] [Google Scholar]
- Uchida A., Micksche M. In vitro augmentation of natural killing activity by OK-432. Int J Immunopharmacol. 1981;3(4):365–375. doi: 10.1016/0192-0561(81)90032-1. [DOI] [PubMed] [Google Scholar]
- Vose B. M., Moore M. Natural cytotoxicity in humans: susceptibility of freshly isolatd tumor cells to lysis. J Natl Cancer Inst. 1980 Aug;65(2):257–263. [PubMed] [Google Scholar]
- Vose B. M., Vanky F., Klein E. Lymphocyte cytotoxicity against autologous tumour biopsy cells in humans. Int J Cancer. 1977 Oct 15;20(4):512–519. doi: 10.1002/ijc.2910200407. [DOI] [PubMed] [Google Scholar]
- Vose B. M., Vánky F., Fopp M., Klein E. Restricted autologous lymphocytotoxicity in lung neoplasia. Br J Cancer. 1978 Sep;38(3):375–381. doi: 10.1038/bjc.1978.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose B. M., Vánky F., Klein E. Human tumour--lymphocyte interaction in vitro. V. Comparison of the reactivity of tumour-infiltrating, blood and lymph-node lymphocytes with autologous tumour cells. Int J Cancer. 1977 Dec 15;20(6):895–902. doi: 10.1002/ijc.2910200612. [DOI] [PubMed] [Google Scholar]
- Vánky F. T., Argov S. A., Einhorn S. A., Klein E. Role of alloantigens in natural killing. Allogeneic but not autologous tumor biopsy cells are sensitive for interferon-induced cytotoxicity of human blood lymphcoytes. J Exp Med. 1980 May 1;151(5):1151–1165. doi: 10.1084/jem.151.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasugi H., Kasahara T., Minato N., Hamuro J., Miyata M., Morioka Y. In vitro potentiation of human natural killer cell activity by a streptococcal preparation, OK-432: interferon and interleukin-2 participation in the stimulation with OK-432. J Natl Cancer Inst. 1982 Oct;69(4):807–812. [PubMed] [Google Scholar]
- Werkmeister J. A., Pihl E., Nind A. P., Flannery G. R., Nairn R. C. Immunoreactivity by intrinsic lymphoid cells in colorectal carcinoma. Br J Cancer. 1979 Dec;40(6):839–847. doi: 10.1038/bjc.1979.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Eskra L., Borden E. C., Horoszewicz J., Carter W. A. Activation of human natural killer cells cytotoxic for human leukemia cells by purified interferon. J Immunol. 1979 Jul;123(1):63–70. [PubMed] [Google Scholar]


