Abstract
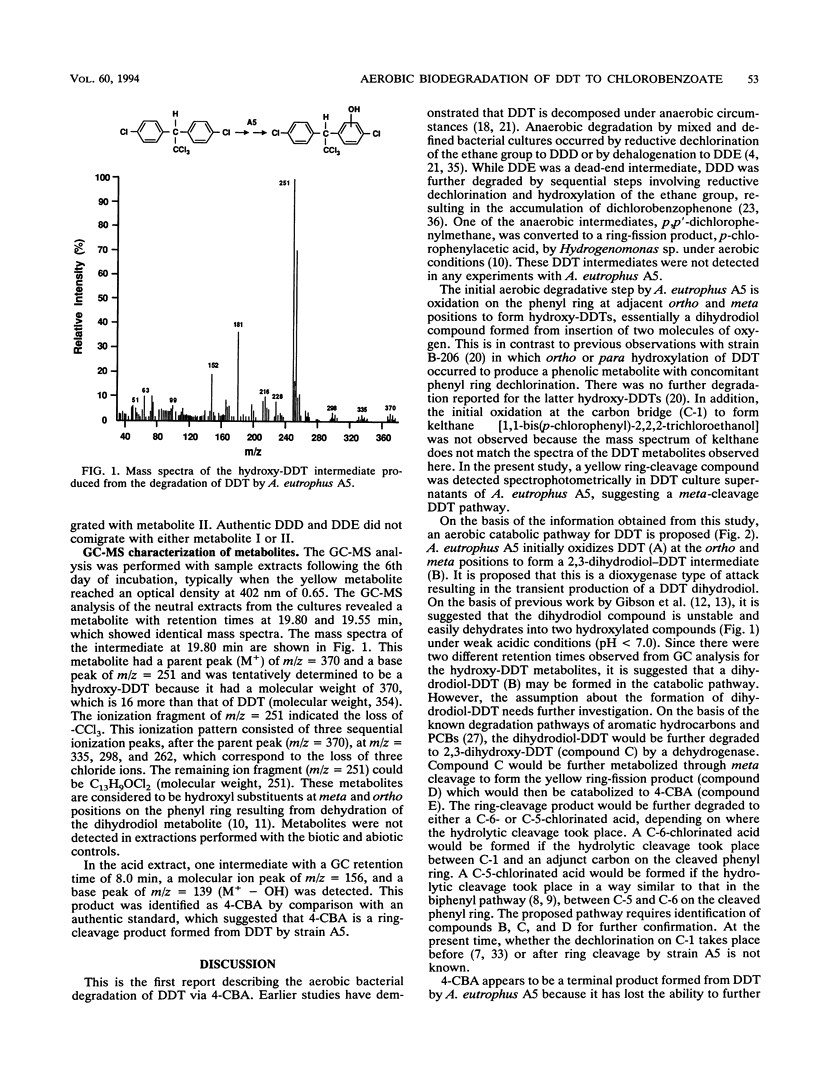
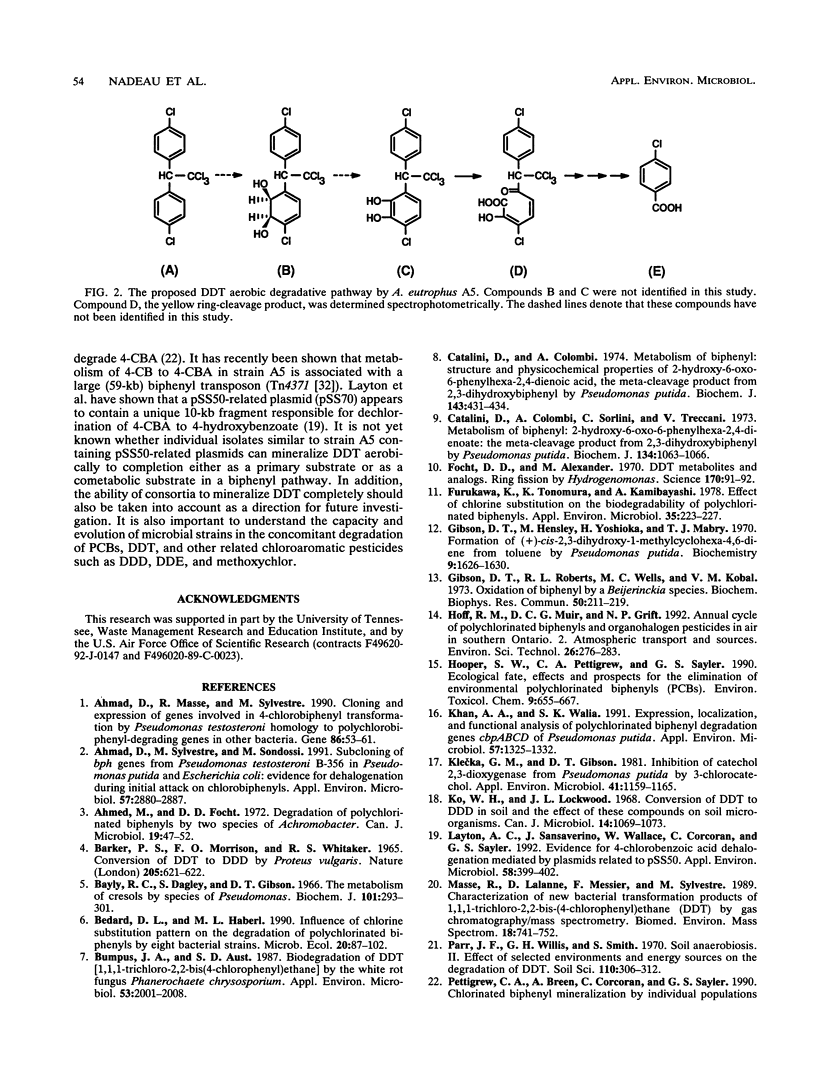
Biotransformation of 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane (DDT) by Alcaligenes eutrophus A5 was demonstrated by analysis of ethyl acetate-extracted products from resting cell cultures. Gas chromatography-mass spectrometry characterization of the neutral extracts revealed two hydroxy-DDT intermediates (m/z = 370) with retention times at 19.55 and 19.80 min that shared identical mass spectra. This result suggested that the hydroxylations occurred at the ortho and meta positions on the aromatic ring. UV-visible spectrum spectrophotometric analysis of a yellow metabolite in the culture supernatant showed a maximum A402 with, under acidic and basic conditions, spectrophotometric characteristics similar to those of the aromatic ring meta-cleavage products. 4-Chlorobenzoic acid was detected by thin-layer chromatography radiochemical scanning in samples from mineralization experiments by comparison of Rf values of [14C]DDT intermediates with that of an authentic standard. These results were further confirmed by gas chromatography-mass spectrometry analysis. This study indicates that DDT appears to be oxidized by a dioxygenase in A. eutrophus A5 and that the products of this oxidation are subsequently subjected to ring fission to eventually yield 4-chlorobenzoic acid as a major stable intermediate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad D., Massé R., Sylvestre M. Cloning and expression of genes involved in 4-chlorobiphenyl transformation by Pseudomonas testosteroni: homology to polychlorobiphenyl-degrading genes in other bacteria. Gene. 1990 Jan 31;86(1):53–61. doi: 10.1016/0378-1119(90)90113-6. [DOI] [PubMed] [Google Scholar]
- Ahmad D., Sylvestre M., Sondossi M. Subcloning of bph genes from Pseudomonas testosteroni B-356 in Pseudomonas putida and Escherichia coli: evidence for dehalogenation during initial attack on chlorobiphenyls. Appl Environ Microbiol. 1991 Oct;57(10):2880–2887. doi: 10.1128/aem.57.10.2880-2887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M., Focht D. D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973 Jan;19(1):47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Aust S. D. Biodegradation of DDT [1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane] by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1987 Sep;53(9):2001–2008. doi: 10.1128/aem.53.9.2001-2008.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelani D., Colombi A. Metabolism of biphenyl. Structure and physicochemical properties of 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid, the meta-cleavage product from 2,3-dihydroxybiphenyl by Pseudomonas putida. Biochem J. 1974 Nov;143(2):431–434. doi: 10.1042/bj1430431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelani D., Colombi A., Sorlini C., Treccani V. Metabolism of biphenyl. 2-Hydroxy-6-oxo-6-phenylhexa-2,4-dienoate: the meta-cleavage product from 2,3-dihydroxybiphenyl by Pseudomonas putida. Biochem J. 1973 Aug;134(4):1063–1066. doi: 10.1042/bj1341063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht D. D., Alexander M. DDT metabolites and analogs: ring fission by Hydrogenomonas. Science. 1970 Oct 2;170(3953):91–92. doi: 10.1126/science.170.3953.91. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Tonomura K., Kamibayashi A. Effect of chlorine substitution on the biodegradability of polychlorinated biphenyls. Appl Environ Microbiol. 1978 Feb;35(2):223–227. doi: 10.1128/aem.35.2.223-227.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Hensley M., Yoshioka H., Mabry T. J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- Khan A. A., Walia S. K. Expression, localization, and functional analysis of polychlorinated biphenyl degradation genes cbpABCD of Pseudomonas putida. Appl Environ Microbiol. 1991 May;57(5):1325–1332. doi: 10.1128/aem.57.5.1325-1332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Inhibition of catechol 2,3-dioxygenase from Pseudomonas putida by 3-chlorocatechol. Appl Environ Microbiol. 1981 May;41(5):1159–1165. doi: 10.1128/aem.41.5.1159-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko W. H., Lockwood J. L. Conversion of DDT to DDD in soil and the effect of these compounds on soil microorganisms. Can J Microbiol. 1968 Oct;14(10):1069–1073. doi: 10.1139/m68-180. [DOI] [PubMed] [Google Scholar]
- Layton A. C., Sanseverino J., Wallace W., Corcoran C., Sayler G. S. Evidence for 4-chlorobenzoic acid dehalogenation mediated by plasmids related to pSS50. Appl Environ Microbiol. 1992 Jan;58(1):399–402. doi: 10.1128/aem.58.1.399-402.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé R., Lalanne D., Messier F., Sylvestre M. Characterization of new bacterial transformation products of 1,1,1-trichloro-2,2-bis-(4-chlorophenyl) ethane (DDT) by gas chromatography/mass spectrometry. Biomed Environ Mass Spectrom. 1989 Sep;18(9):741–752. doi: 10.1002/bms.1200180917. [DOI] [PubMed] [Google Scholar]
- Pettigrew C. A., Breen A., Corcoran C., Sayler G. S. Chlorinated biphenyl mineralization by individual populations and consortia of freshwater bacteria. Appl Environ Microbiol. 1990 Jul;56(7):2036–2045. doi: 10.1128/aem.56.7.2036-2045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaender F. K., Alexander M. Extensive microbial degradation of DDT in vitro and DDT metabolism by natural communities. J Agric Food Chem. 1972 Jul-Aug;20(4):842–846. doi: 10.1021/jf60182a045. [DOI] [PubMed] [Google Scholar]
- Quensen J. F., 3rd, Tiedje J. M., Boyd S. A. Reductive dechlorination of polychlorinated biphenyls by anaerobic microorganisms from sediments. Science. 1988 Nov 4;242(4879):752–754. doi: 10.1126/science.242.4879.752. [DOI] [PubMed] [Google Scholar]
- Robinson J., Richardson A., Crabtree A. N., Coulson J. C., Potts G. R. Organochlorine residues in marine organisms. Nature. 1967 Jun 24;214(5095):1307–1311. doi: 10.1038/2141307a0. [DOI] [PubMed] [Google Scholar]
- Sayler G. S., Lund L. C., Shiaris M. P., Sherrill T. W., Perkins R. E. Comparative effects of Aroclor 1254 (polychlorinated biphenyls) and phenanthrene on glucose uptake by freshwater microbial populations. Appl Environ Microbiol. 1979 May;37(5):878–885. doi: 10.1128/aem.37.5.878-885.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields M. S., Hooper S. W., Sayler G. S. Plasmid-mediated mineralization of 4-chlorobiphenyl. J Bacteriol. 1985 Sep;163(3):882–889. doi: 10.1128/jb.163.3.882-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springael D., Kreps S., Mergeay M. Identification of a catabolic transposon, Tn4371, carrying biphenyl and 4-chlorobiphenyl degradation genes in Alcaligenes eutrophus A5. J Bacteriol. 1993 Mar;175(6):1674–1681. doi: 10.1128/jb.175.6.1674-1681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Rao R. V., Alexander M. Bacterial and fungal cometabolism of 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane (DDT) and its breakdown products. Appl Environ Microbiol. 1985 Mar;49(3):509–516. doi: 10.1128/aem.49.3.509-516.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre M. Isolation Method for Bacterial Isolates Capable of Growth on p-Chlorobiphenyl. Appl Environ Microbiol. 1980 Jun;39(6):1223–1224. doi: 10.1128/aem.39.6.1223-1224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedemeyer G. Dechlorination of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane by Aerobacter aerogenes. I. Metabolic products. Appl Microbiol. 1967 May;15(3):569–574. doi: 10.1128/am.15.3.569-574.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedemeyer G. Dechlorination of DDT by Aerobacter aerogenes. Science. 1966 Apr 29;152(3722):647–647. doi: 10.1126/science.152.3722.647. [DOI] [PubMed] [Google Scholar]
- Yates J. R., Mondello F. J. Sequence similarities in the genes encoding polychlorinated biphenyl degradation by Pseudomonas strain LB400 and Alcaligenes eutrophus H850. J Bacteriol. 1989 Mar;171(3):1733–1735. doi: 10.1128/jb.171.3.1733-1735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


