Abstract
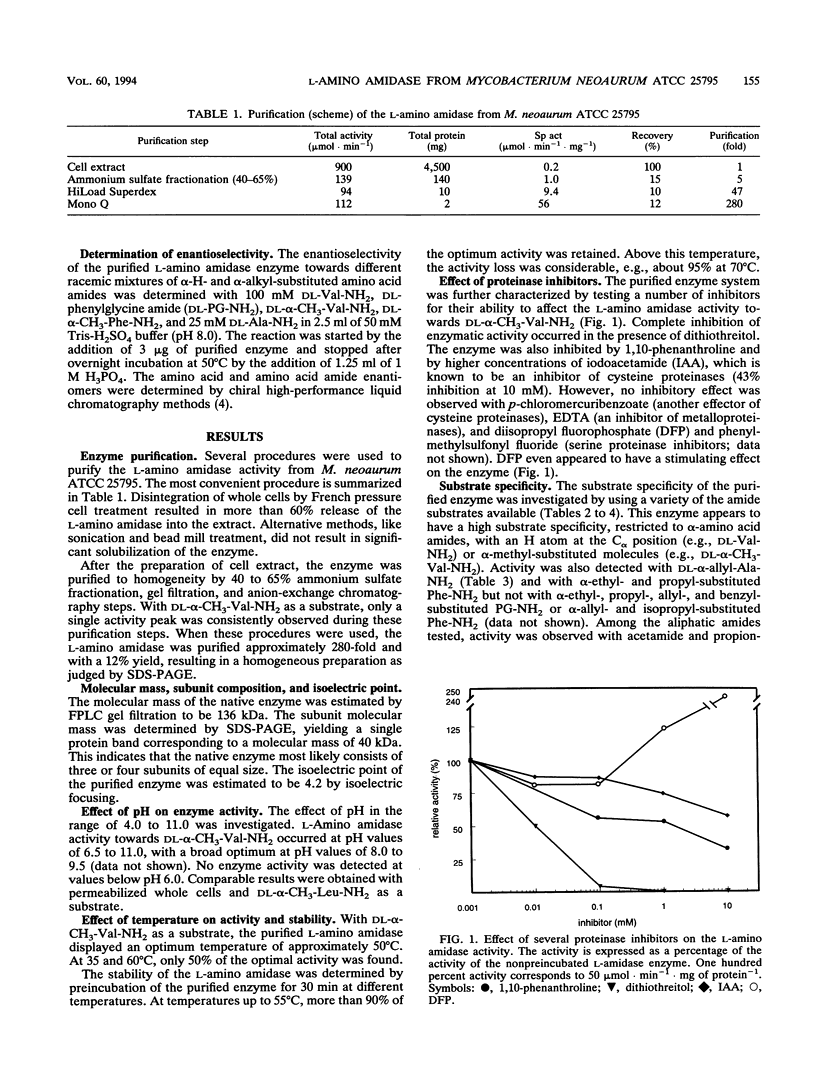
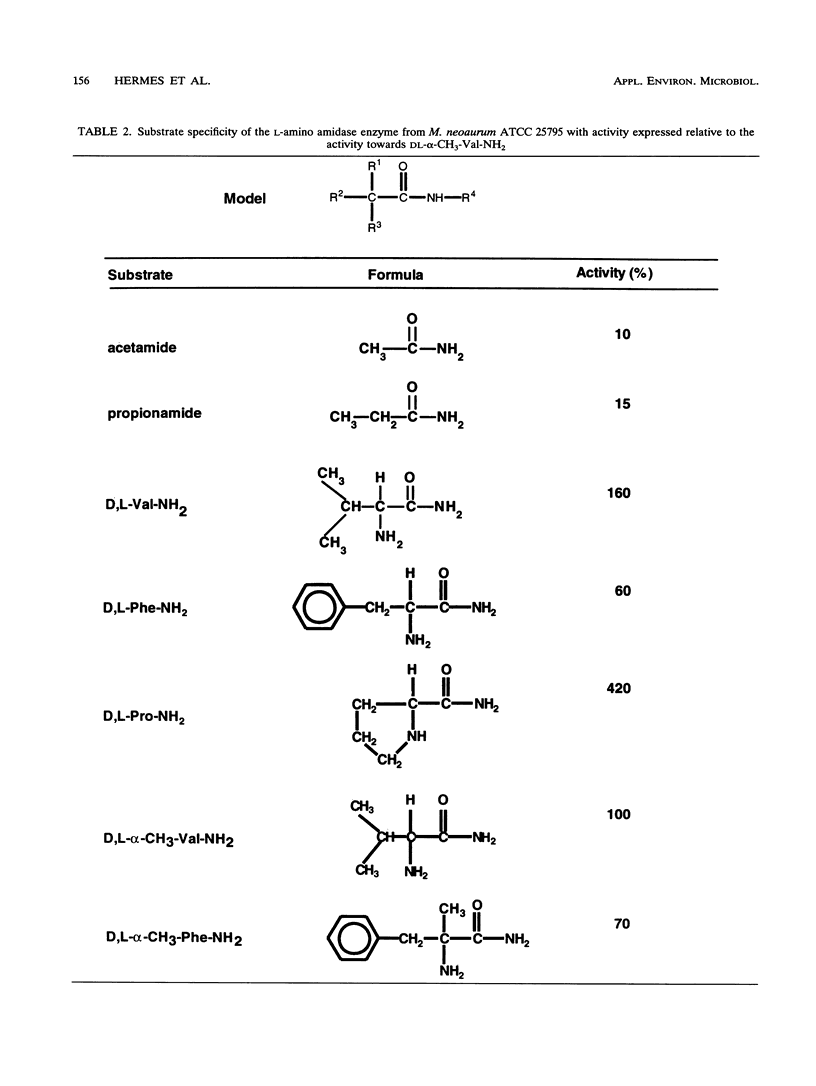
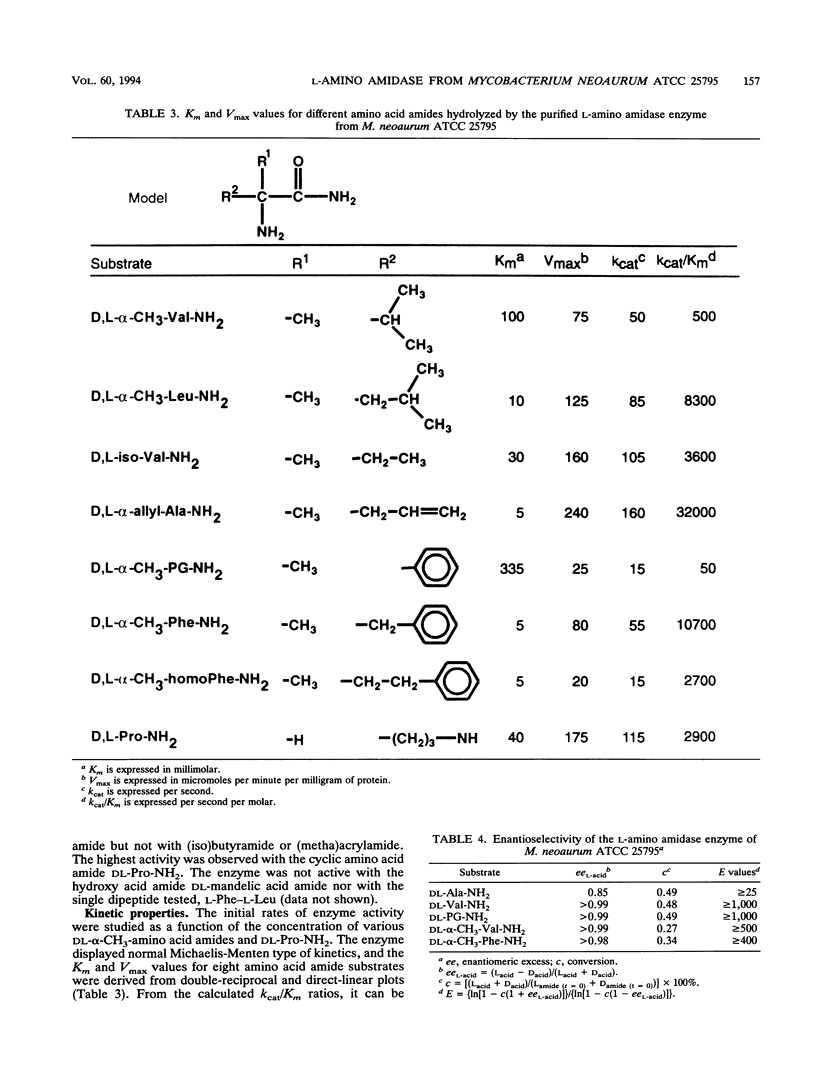
An l-amino amidase from Mycobacterium neoaurum ATCC 25795 responsible for the enantioselective resolution of dl-α-methyl valine amide was purified and characterized. The purification procedure included ammonium sulfate fractionation, gel filtration, and anion-exchange chromatography, which resulted in a homogeneous preparation of the enzyme with a native molecular mass of 136 kDa and a subunit molecular mass of 40 kDa. The purified enzyme displayed the highest activity at 50°C and at pH 8.0 and 9.5. The enzyme was strongly inhibited by the metal-chelating agent 1,10-phenanthroline, the disulfide-reducing agent dithiothreitol, and the cysteine proteinase inhibitor iodoacetamide. The purified amino amidase showed a unique l-enantioselective activity towards a broad range of both α-H- and α-alkyl-substituted amino acid amides, with the highest activity towards the cyclic amino acid amide dl-proline amide. No activity was measured with dl-mandelic acid amide nor with the dipeptide l-phenylalanine-l-leucine. The highest catalytic efficiency (kcat/Km ratio) was measured with dl-α-allyl alanine amide, dl-α-methyl phenylalanine amide, and dl-α-methyl leucine amide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Fox R. O., Jr, Richards F. M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature. 1982 Nov 25;300(5890):325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]
- Goodman M. Peptide homologs, isosteres, and isomers: a general approach to structure-activity relationships. Biopolymers. 1985 Jan;24(1):137–155. doi: 10.1002/bip.360240112. [DOI] [PubMed] [Google Scholar]
- Kalisz H. M. Microbial proteinases. Adv Biochem Eng Biotechnol. 1988;36:1–65. doi: 10.1007/BFb0047944. [DOI] [PubMed] [Google Scholar]
- Kamphuis J., Boesten W. H., Broxterman Q. B., Hermes H. F., van Balken J. A., Meijer E. M., Schoemaker H. E. New developments in the chemo-enzymatic production of amino acids. Adv Biochem Eng Biotechnol. 1990;42:133–186. doi: 10.1007/BFb0000733. [DOI] [PubMed] [Google Scholar]
- Maestracci M., Bui K., Thiéry A., Arnaud A., Galzy P. The amidases from a Brevibacterium strain: study and applications. Adv Biochem Eng Biotechnol. 1988;36:67–115. doi: 10.1007/BFb0047945. [DOI] [PubMed] [Google Scholar]
- Pandey R. C., Meng H., Cook J. C., Jr, Rinehart K. L., Jr Structure of antiamoebin I from high resolution field desorption and gas chromatographic mass spectrometry studies. J Am Chem Soc. 1977 Jul 20;99(15):5203–5205. doi: 10.1021/ja00457a063. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]


