Abstract
A major line of evidence that supports the hypothesis of dopamine overactivity in schizophrenia is the psychomimetic potential of agents such as amphetamine that stimulate dopamine outflow. A novel brain imaging method provides an indirect measure of in vivo synaptic dopamine concentration by quantifying the change in dopamine receptor radiotracer binding produced by agents that alter dopamine release but do not themselves bind to dopamine receptors. The purpose of this investigation is (i) to determine the sensitivity (i.e., amount of dopamine reflected in radiotracer binding changes) of this method by examining the relationship between amphetamine-induced changes in simultaneously derived striatal extracellular dopamine levels with in vivo microdialysis and striatal binding levels with the dopamine D2/D3 positron-emission tomography radioligand [11C]raclopride in nonhuman primates, and (ii) to test the hypothesis of elevated amphetamine-induced synaptic dopamine levels in schizophrenia. In the nonhuman primate study (n = 4), doubling the amphetamine dose produced a doubling in [11C]raclopride specific binding reductions. In addition, the ratio of percent mean dopamine increase to percent mean striatal binding reduction for amphetamine (0.2 mg/kg) was 44:1, demonstrating that relatively small binding changes reflect large changes in dopamine outflow. In the clinical study, patients with schizophrenia (n = 11) compared with healthy volunteers (n = 12) had significantly greater amphetamine-related reductions in [11C]raclopride specific binding (mean ± SEM): −22.3% (±2.7) vs. −15.5% (±1.8), P = 0.04, respectively. Inferences from the preclinical study suggest that the patients’ elevation in synaptic dopamine concentrations was substantially greater than controls. These data provide direct evidence for the hypothesis of elevated amphetamine-induced synaptic dopamine concentrations in schizophrenia.
Keywords: raclopride, brain imaging, striatum, in vivo microdialysis, psychostimulants
Dopamine overactivity has been the predominant pathophysiologic hypothesis of schizophrenia for the past two decades (1–3). A major line of evidence used to support dopamine’s involvement in schizophrenia is the psychotomimetic effects of agents that stimulate dopamine outflow, such as the psychostimulant amphetamine. Psychostimulants can produce a paranoid psychosis in healthy individuals (4) and cause symptomatic worsening in approximately one-third of patients with schizophrenia (5). These data support the hypothesis that at least a subgroup of schizophrenia is associated with increased synaptic dopamine concentrations. This hypothesis, however, could not be directly tested because, prior to the introduction of the experimental paradigm described here (6, 7), there has been no method to quantify in vivo synaptic dopamine levels in clinical populations.
A relatively new application of in vivo brain imaging provides an estimate of changes in synaptic dopamine concentrations. This approach determines the change in striatal radiotracer binding levels following administration of pharmacologic agents that affect dopamine outflow but do not themselves bind to dopamine receptors (6, 7). The change in striatal radiotracer binding levels is attributable to changes in synaptic dopamine that competes with the radiotracer for receptor binding. In a recently published report using the D2 ligand 123I-IBZM (iodobenzamide) and single photon emission tomography (SPECT), Laruelle et al. (8) found that schizophrenic patients compared with controls had significantly greater reductions in amphetamine-induced striatal radiotracer binding ratios, which supports enhanced dopamine outflow. Although this method has undergone extensive validation (6, 7, 9–12), there has yet to be a direct comparison between simultaneous changes in extracellular dopamine levels and changes in radiotracer binding levels in mammalian brain. These data would provide an important measure of sensitivity (i.e., amount of dopamine reflected in radiotracer binding changes), which would allow inferences about the magnitude of dopamine responses in clinical populations.
In this paper, we report results from two experiments. The first examined the relationship between changes in extracellular striatal dopamine and striatal dopamine receptor radiotracer binding in nonhuman primates. The effects of two doses of amphetamine (0.2 and 0.4 mg/kg) on extracellular striatal dopamine levels derived with in vivo microdialysis and striatal dopamine receptor radiotracer binding levels determined with positron-emission tomography (PET) were assessed simultaneously. Our brain imaging approach involved a constant infusion of the PET radiotracer [11C]raclopride (13, 14) until tracer equilibrium was reached. [11C]raclopride has high selectivity and low affinity for dopamine D2/D3 receptors and avidly competes with synaptic dopamine for receptor occupancy (15–19). Then, amphetamine was administered which increases synaptic dopamine which in turn displaces [11C]raclopride striatal binding. In the second experiment, we employed this brain imaging method to test the hypothesis that schizophrenic patients, in comparison to healthy controls, have greater amphetamine-induced striatal synaptic dopamine concentrations. Data from the first experiment were used to draw inferences about the magnitude of dopamine response differences between controls and patients.
MATERIALS AND METHODS
In Vivo Microdialysis/PET Study. Subjects.
Four adult rhesus monkeys (Macaca mulatta) were used in this study (weight, 7.4–11.2 kg). They were housed individually on a 12-hr light/dark cycle. Food and water were available ad libitum. All procedures were carried out with strict adherence to the NIH Guide for the Care and Use of Laboratory Animals and had been approved by the National Institute of Mental Health Animal Care and Use Committee.
Microdialysis Probes and Probe Placement.
The microdialysis probes were constructed as described earlier (20, 21). Briefly, we used two fused silica barrels, the inner barrel extending 3–5 mm beyond the distal end of the outer barrel. A short piece (4–6 mm) of dialysis membrane tubing (AN 69 polyacrylonitrile membrane, 240 mm i.d., 300 mm o.d., 40,000 Mr cutoff; Hospal Dasco, Bologna, Italy) was placed over the extended portion of the inner barrel and glued to the inside surface of the outer barrel using cyanoacrylate instant adhesive. All probes were tested in vitro for dopamine recovery. The probes were placed in a beaker containing 10–7 M dopamine (Sigma) in artificial cerebrospinal fluid buffered with 1.0 mM phosphate (pH 7.4) and supplemented with 0.15 mM ascorbate at 37°C. The probes were perfused at 1 μl/min flow rate for 2 hr before three 25-min collections were made for chromatographic analysis of recovered dopamine utilizing electrochemical detection. The mean recovery from three collections of each probe was considered as the criteria for selecting a probe for in vivo dialysis, with recovery values >30% used for in vivo microdialysis (21, 22). Stereotactic coordinates for the head of the caudate nucleus were determined for individual animals using MRI (23). Specially constructed guide holders were fixed to the skull under sterile surgical conditions to position and secure the probes into the targeted brain regions (24). The animals were allowed to recover for 2–3 weeks prior to any in vivo microdialysis experiments.
In Vivo Microdialysis.
On the morning of each study, monkeys were initially sedated with ketamine (ketamine hydrochloride, 10 mg/kg, i.m.), intubated, and anesthetized with gas isofluorane (1–3%). Animals were kept under light gaseous anesthesia throughout the procedure. Each animal was wrapped in a heating blanket (37°C) and vital signs (heart rate, temperature, and respiration) were monitored throughout the experimental session. Two probes were placed into the head of the caudate nucleus. Artificial cerebrospinal fluid (CSF; 147 mM Na+, 3 mM K+, 1.3 mM Ca2+, 1.0 mM Mg2+, 155 mM Cl−, buffered with 1.0 mM phosphate, pH 7.3–7.4) supplemented with 0.15 mM ascorbate was continuously perfused through the probes at 1.5 μl/min flow rate using a Harvard microinfusion pump (Harvard Apparatus). Dialysate samples (15 μl) were collected every 10 min in amber-colored glass vials and immediately frozen on dry ice. They were assayed for dopamine using microbore HPLC coupled to electrochemical detection (21). Analytical reagent-grade chemicals were used for preparation of artificial CSF (Mallinckrodt).
PET Procedure.
Animal studies were conducted on a Scanditronix (Uppsala) model PC 2048–15B scanner. Animals were positioned in a head holder developed for nonhuman primate PET studies, and slices were obtained in the coronal plane. Following a transmission scan, [11C]raclopride (2–6 mCi; 1 Ci = 37 GBq) was administered in a bolus/constant infusion over 90 min (25). The bolus dose was equivalent to 40% of the total dose administered and produced nearly constant radioactivity levels by 25–30 min postinjection. Forty minutes after the raclopride bolus, the first dose of amphetamine, 0.2 mg/kg, was administered i.v. Beginning with the raclopride bolus, 31 PET scans (15 slices each; in-plane resolution, 7 mm; slice width, 6 mm) were obtained over 90 min, which concluded the first raclopride study. There was then an ≈90-min rest period until the second raclopride study commenced. The second study involved repeating the procedure from the first study except that 0.4 mg/kg of amphetamine was administered 40 min after the second raclopride bolus.
Dopamine Data Analysis.
Dopamine response was determined by percent change from baseline. Baseline for both 0.2 and 0.4 mg/kg amphetamine studies was determined from a mean of four consecutive samples collected immediately preceding the 0.2 mg/kg amphetamine administration. Postamphetamine dopamine effects were determined using a mean of four consecutive samples following the 0.2 and 0.4 mg/kg amphetamine administrations.
PET Data Processing and Analysis.
Image processing was performed with mirage software developed by the National Institutes of Health PET Center. The images corresponding to 0–5 min of raclopride infusion were added together to form a single “sum” image. Volumes of interest (VOIs) were drawn in the cerebellum and on the left and right striatum (consisting of caudate and putamen combined). After visual inspection, these VOIs were then overlaid onto their corresponding position in each of the 31 individual scans, and samples (mean pixel values) were generated for each VOI. Left and right striatal VOIs were averaged to a single striatal value. These data from five consecutive scans 25–40 min after injection and immediately before amphetamine injection (baseline) and five consecutive scans 65–90 min post-raclopride bolus administration (postamphetamine) were averaged. The specific binding was computed as striatum/cerebellum−1 (26).
Clinical Study. Subject Characteristics.
All subjects gave informed written consent to an institutional review board-approved protocol. Eleven patients with schizophrenia disorder diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, 4th Ed. (DSM-IV) criteria participated in the study. Diagnoses were determined by a diagnostic conference utilizing data from a structured diagnostic interview (SCID) (27), clinical interview by a research psychiatrist, past psychiatric and medical records, and informant interviews. Patients were excluded if they had a history of illegal drug dependance and/or significant drug abuse, severe head trauma resulting in loss of consciousness, and any medical condition that made amphetamine administration contraindicated. The schizophrenic patients were ill for (mean ± SEM) 6.6 ± 1.8 years prior to study participation. Six of the 11 patients had either no or minimal previous antipsychotic drug exposure and were termed the neuroleptic-naive subgroup (four patients had no antipsychotic drug exposure and two had less than 1 week of low-dose neuroleptic exposure occurring more than 3 years before entry into the study). The remaining five were withdrawn from antipsychotic treatment prior to participation (days drug-free, mean ± SEM, 23.2 ± 7.2 days; range, 14–48 days). The control group was 12 healthy volunteers who were free of past and current psychiatric disorders as determined by a structured diagnostic interview. There were no significant differences (P > 0.4, mean ± SEM) between schizophrenics and controls, respectively, for age (32.4 ± 3.0 vs. 29.2 ± 2.6 years), gender (M/F, 8/3 vs. 9/3), and body weight (85.6 ± 3.8 vs. 80.0 ± 7.0 kg).
PET Scanning Protocol.
Clinical studies were conducted on a General Electric (GE) Advance scanner at the National Institutes of Health Clinical Center. Acquisitions were done with the interplane septa retracted and a wide axial acceptance angle. Each scan yielded 35 planes, 4.25 mm apart. The effective resolution of the reconstruction was 6 mm both axially and in-plane. A transmission scan was performed using two rotating 68Ge sources and was used for attenuation correction.
Subjects were positioned in the scanner such that acquired planes would be parallel to the orbital-meatal line. Head movement was minimized with individually fitted thermoplaster masks, and patches were applied over the orbits to reduce incoming light. [11C]raclopride (2–8 mCi) was administered as bolus/constant infusion over 2 hr. The bolus dose was 53% of the total amount administered. Beginning with the raclopride bolus, 29 scans were acquired over the 2-hr period every 3–5 min. Fifty minutes after commencement of raclopride administration, amphetamine (0.2 mg/kg i.v.) was infused over 60 sec. A 50-min baseline period (as opposed to 40 min used in experiment 1) was used because it provided a longer tracer equilibrium period. A longer scanning period was permitted by the enhanced sensitivity of the GE Advance scanner compared with the Scanditronix used in the monkey study. The dose of amphetamine was selected based on the nonhuman primate data that demonstrated robust increases in dopamine levels (see below) and because it was well tolerated in terms of cardiovascular and behavioral effects in schizophrenic patients. For ethical and safety reasons, we elected not to use higher doses so that the likelihood of inducing a psychotic exacerbation was minimized. Plasma samples for amphetamine levels were drawn 40 min after amphetamine administration.
Behavioral responses were examined with the Brief Psychiatric Rating Scale (BPRS; ref. 28). The BPRS is a commonly used clinical rating scale that is composed of 18 different psychiatric symptoms. Four of the items are psychotic symptoms (i.e., hallucinations, paranoia, delusions, and conceptual disorganization) and are grouped together as the BPRS psychosis subfactor. Both the BPRS total score, reflecting global symptomatology, and the BPRS psychosis subfactor score were used in this study. The scale was administered in a clinical interview by a research psychiatrist at baseline (before raclopride administration), 15 min after amphetamine administration (during-drug), and at the end of the study. The BPRS total score and the psychosis subfactor score were derived by summing each symptom (rated from 1, not present, to 7, severe). During-drug minus baseline change scores were used for correlative analyses with changes in raclopride binding ratios.
Data Processing and Analysis.
Image processing and PET data analysis were performed as described in the preclinical study with the following exceptions. Ratio data from five consecutive scans 30–50 min after injection and immediately before amphetamine administration (baseline) and five consecutive scans 75–100 min post-raclopride bolus injection (postamphetamine) were averaged (see Fig. 1). Individual group comparisons were conducted with paired and unpaired t tests where appropriate. Behavioral data (BPRS total score and psychosis subfactor score) were analyzed with a repeated-measures ANOVA with “time” (i.e., baseline, during-drug, and end-of-study ratings) and “group” (i.e., schizophrenics vs. controls) as factors. Change in behavior (from baseline to during-drug) were correlated with change in percent [11C]raclopride striatal binding ratios using a Pearson’s correlation coefficient. All comparisons were two-tailed and group data were presented as mean ± SEM.
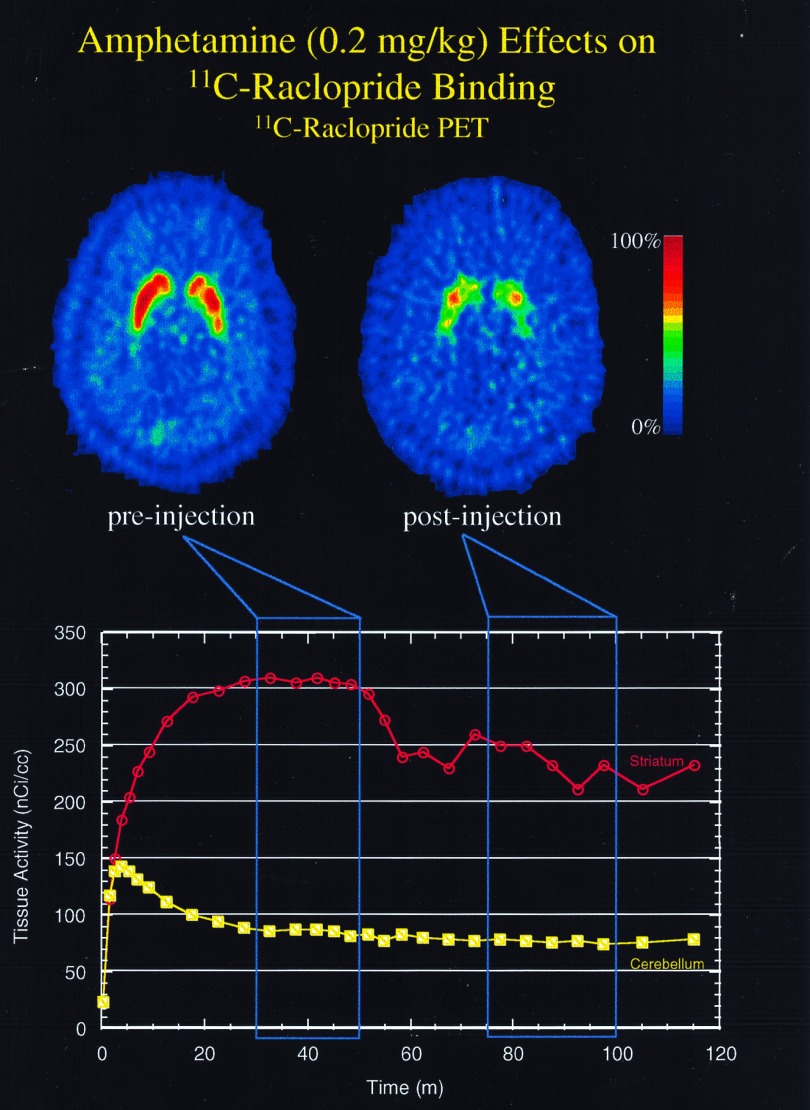
Figure 1.
The effects of amphetamine (0.2 mg/kg) on [11C]raclopride binding in a patient with schizophrenia. Amphetamine was injected at 50 min. (Upper) “Sum” images of five [11C]raclopride PET scans obtained before (Left) and after (Right) amphetamine injection. (Lower) Time-activated curves obtained in the striatum (green) and cerebellum (blue) with a bolus/constant infusion of [11C]raclopride. This patient had a −20.6% reduction in pre- vs. post-[11C]raclopride specific binding (striatum/cerebellum−1).
RESULTS
In Vivo Microdialysis/PET Study.
Amphetamine (0.2 mg/kg) increased striatal dopamine levels 459% (±221) from baseline levels and 0.4 mg/kg caused increases of 1365% (±485) (Table 1). Simultaneously derived pre- and poststriatal [11C]raclopride binding ratios for 0.2 mg/kg were 2.0 ± 0.1 and 1.0 ± 0.2, respectively; and for 0.4 mg/kg were 1.9 ± 0.1 and 1.5 ± 0.2, respectively. Percent reductions in binding ratios for the two amphetamine doses were 10.5% (±5.4) and 21.3% (±5.4), respectively (Table 2). Thus, doubling amphetamine dose caused an approximate doubling in mean striatal binding reductions. The magnitude of dopamine responses was several-fold greater than the corresponding reductions in [11C]raclopride binding. The ratio of mean percent dopamine increases to mean percent binding reductions was 44:1 for 0.2 mg/kg, and 64:1 for 0.4 mg/kg amphetamine. The range in amphetamine-induced increases from baseline among the four monkeys for 0.2 mg/kg was 84.1–1006.9%, and for 0.4 mg/kg was 288.5–2470.7%. Thus, the most conservative ratio based on data from the monkey with the lowest dopamine pulse for 0.2 mg/kg and a binding reduction of 10.5% was 8:1 and for 0.4 mg/kg and a binding reduction of 21.3% was 14:1. Using a compartmental model that incorporated the in vivo microdialysis data, we confirmed a linear relationship between amphetamine-related dopamine pulses and striatal binding changes (29).
Table 1.
Effects of two doses of amphetamine (0.2 and 0.4 mg/kg) on striatal extracellular dopamine levels (nmol/liter) in four Rhesus monkeys
| Monkey | Baseline | 0.2 mg/kg | % baseline | 0.4 mg/kg | % baseline |
|---|---|---|---|---|---|
| 1 | 5.9 | 43.6 | +632.2 | 114.4 | +1820.5 |
| 2 | 4.6 | 51.6 | +1006.9 | 120.0 | +2470.7 |
| 3 | 6.6 | 14.4 | +116.5 | 65.4 | +881.3 |
| 4 | 4.5 | 8.3 | +84.1 | 17.6 | +288.5 |
| Mean (±SEM) | 5.4 (±0.5) | 29.5 (±10.6) | +459.9* (±221.4) | 79.3 (±23.9) | +1365.2* (±485.0) |
t = 3.3, P = 0.045; % baseline, 0.2 mg/kg vs. % baseline, 0.4 mg/kg.
Table 2.
Effects of two doses of amphetamine (0.2 and 0.4 mg/kg) on [11C]raclopride binding ratios (striatum/cerebellum −1) in four Rhesus monkeys
| Monkey | Baseline | 0.2 mg/kg | % baseline | Baseline | 0.4 mg/kg | % baseline |
|---|---|---|---|---|---|---|
| 1 | 1.9 | 1.6 | −17.5 | 1.8 | 1.2 | −33.3 |
| 2 | 2.0 | 2.1 | +4.9 | 1.9 | 1.8 | −7.8 |
| 3 | 1.7 | 1.4 | −19.2 | 1.6 | 1.2 | −25.5 |
| 4 | 2.3 | 2.1 | −10.1 | 2.1 | 1.7 | −18.4 |
| Mean (±SEM) | 2.0 (±0.1) | 1.8 (±0.1) | −10.5* (±5.5) | 1.8 (±0.101) | 1.4 (±0.1) | −21.3* (±5.4) |
t = 5.0, P = 0.015; % baseline, 0.2 mg/kg vs. % baseline, 0.4 mg/kg.
Clinical Study.
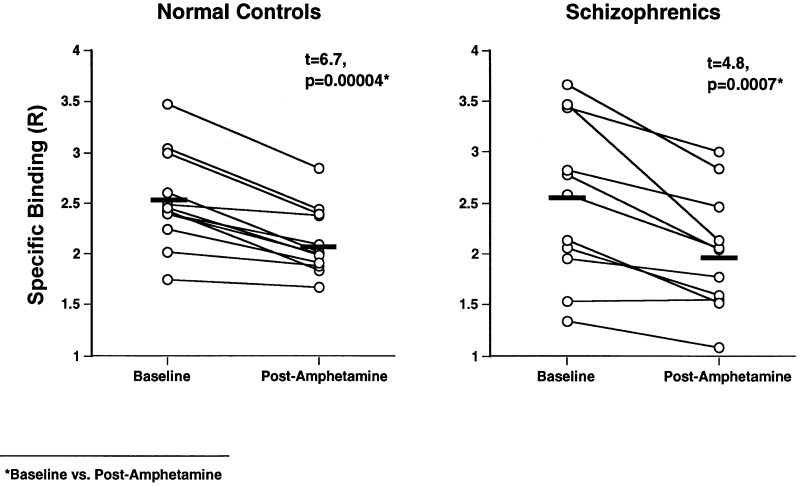
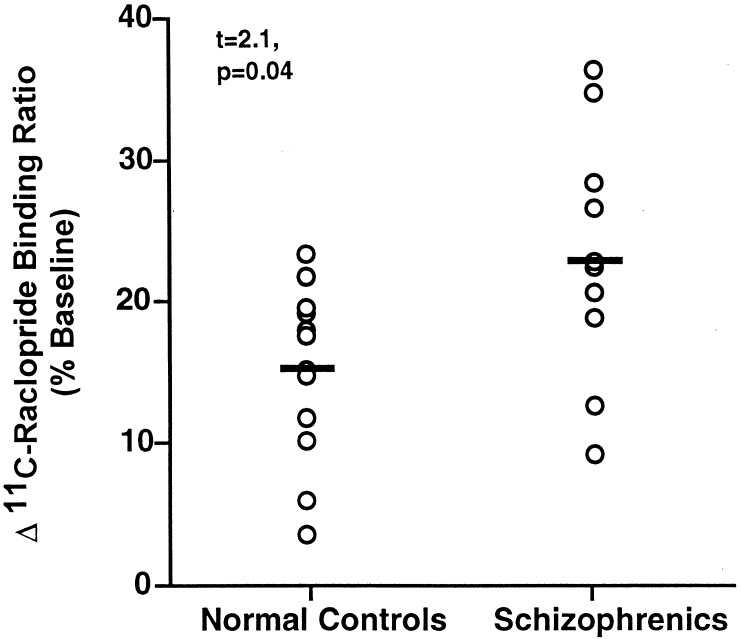
Amphetamine produced highly significant decreases in [11C]raclopride striatal binding ratios (baseline vs. postamphetamine levels) in both healthy controls (2.5 ± 0.1 vs. 2.1 ± 0.1; t = 6.7, P = 0.00004) and schizophrenic patients (2.5 ± 0.2 vs. 2.0 ± 0.1; t = 4.8, P = 0.0007) (Fig. 2). There were no significant differences in baseline striatal binding ratios between controls and schizophrenic patients (t = 0.02, P = 0.99). Schizophrenic patients had greater amphetamine-induced changes in [11C]raclopride striatal binding than controls. The patients had a 22.3% (±2.7) reduction in [11C]raclopride specific striatal binding from baseline to postamphetamine compared with a 15.5% (±1.8) reduction in healthy controls (t = 2.1, P = .04) (Fig. 3). Similar results were found when the groups were compared with nonparametric analysis (Mann–Whitney U Test; z = 2.0, P = 0.048). There were no significant differences in plasma amphetamine levels (ng/ml) between controls (55.3 ± 4.3) and schizophrenic patients (59.3 ± 4.7; t = 0.6, P = 0.5), and plasma levels were not significantly correlated with percent mean binding changes. In addition, there were no significant differences in amphetamine-induced percent change in [11C]raclopride striatal binding between the subgroups of six neuroleptic-naive (24.1%, ±5.0) and five neuroleptic withdrawn (20.3%, ±4.7); t = 0.6, P = 0.5) schizophrenic patients indicating that previous neuroleptic treatment was not responsible for binding differences between schizophrenic patients and controls.
Figure 2.
The effects of amphetamine (0.2 mg/kg) on striatal [11C]raclopride specific binding in normal controls (n = 12) and patients with schizophrenia (n = 11). The data are presented as [11C]raclopride specific binding ratios (striatum/cerebellum−1).
Figure 3.
A comparison between amphetamine (0.2 mg/kg)-induced changes in [11C]raclopride specific binding in normal controls (n = 12) and patients with schizophrenia (n = 11). Data are presented as percent change from baseline in striatal binding ratios (striatum/cerebellum − 1).
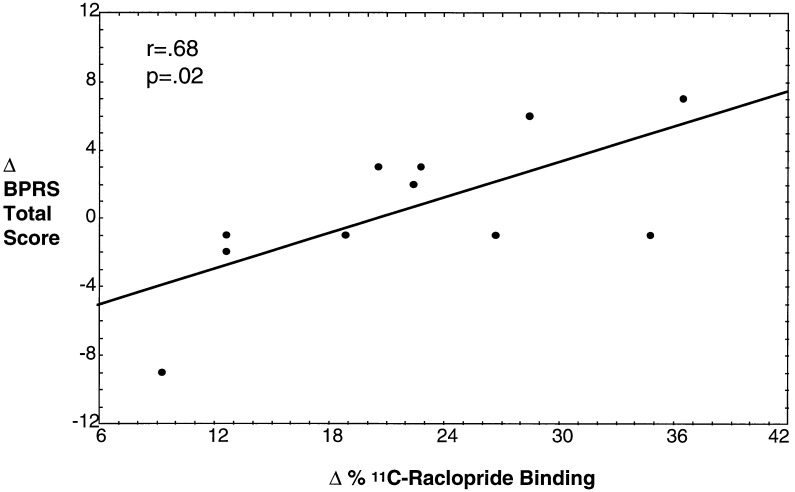
Analysis of BPRS total score (mean ± SEM; baseline, during-drug, and end of study, respectively) for controls (18.1 ± 0.3, 22.2 ± 2.7, and 18.3 ± 0.5) and patients (28.8 ± 7.2, 29.4 ± 6.6, and 27.5 ± 9.8) revealed a significant “time” effect (F = 7.8, P = 0.001) but not a significant “time vs. group” interaction (F = 2.5, P = 0.1). The time effect indicates amphetamine affected global symptomatology, and the lack of a significant interaction indicates the groups did not differ in their responses to amphetamine. For the BPRS psychosis subfactor for controls (4.0 ± 0.0, 4.4 ± 0.7, and 4.0 ± 0.0) and patients (6.7 ± 2.8, 6.8 ± 2.8, and 6.5 ± 2.2), there were no significant “time” (F = 1.4, P = 0.3) or “time vs. group” (F = 0.3, P = 0.8) effects. Only one schizophrenic patient and no controls had increases of 2 points or more on any one of the four items comprising the BPRS psychosis subfactor. Percent change in [11C]raclopride striatal binding ratios were positively correlated with change in BPRS total symptom scores in the patients (Fig. 4) but not controls, and significant correlations with the psychosis subfactor change scores were not found in either patients or controls.
Figure 4.
The relationship between amphetamine (0.2 mg/kg)-induced changes in percent [11C]raclopride specific binding and behavioral effects in schizophrenia patients (n = 11). Behavioral effects were assessed pre- and postamphetamine administration using the total score of BPRS.
DISCUSSION
The results of our clinical study indicated that patients with schizophrenia have significantly greater decrements in amphetamine-related [11C]raclopride striatal binding than healthy controls. The preclinical study provides data suggesting that the patients’ amphetamine-induced elevation in synaptic dopamine concentration is substantially greater than controls. These data support the hypothesis that schizophrenia is associated with increased psychostimulant-induced synaptic dopamine concentrations.
The clinical data are similar to a recently published report by Laruelle et al. (8), who, using the D2 ligand 123I-IBZM and SPECT, found that schizophrenic patients compared with controls had significantly greater reductions in amphetamine-induced striatal radiotracer binding ratios. Approximately one-third of schizophrenic patients in our study (4 of 11) and in the Laruelle study (6 of 15) had striatal binding ratios that were greater and nonoverlapping with the total normal control range, demonstrating agreement between the studies. These data are consistent with the clinical literature that indicates psychostimulants (i.e., amphetamine and methylphenidate) worsen symptoms in approximately one-third of schizophrenic patients, while the remainder of patients either did not change or clinically improved (5). Taken together, these data suggest the possibility that there may be a subgroup of perhaps one-third of schizophrenic patients who have enhanced amphetamine-induced synaptic dopamine concentrations. Our finding is not secondary to prior antipsychotic drug exposure because the neuroleptic-naive subgroup had [11C]raclopride binding decrements that were not significantly different from the schizophrenic patients withdrawn from neuroleptic treatment. In addition, gender, age, weight, and amphetamine blood level cannot explain the findings because the groups were well matched for these variables.
Several caveats must be considered before drawing inferences from the preclinical study to assist in interpreting the clinical data. First, in vivo microdialysis measures extracellular dopamine levels, and changes in synaptic dopamine concentrations are reflected in radiotracer binding changes. In addition, extrapolations from monkey to human, the small sample size, and the large degree of individual variability in dopamine responses limit the generalizability of the data to clinical populations. Nonetheless, our data are consistent with previous reports indicating that amphetamine produces a rapid and robust increase in dopamine outflow in rodents (30, 31) and monkeys (21, 32). We have further demonstrated that very large increases in synaptic dopamine concentrations are reflected in comparatively smaller changes in [11C]raclopride binding. The ratio of mean percent dopamine increases to mean percent binding reductions was 44:1 for amphetamine (0.2 mg/kg). This high ratio may be related, in part, to radiolabeled extrasynaptic D2 receptors, which may be less sensitive to displacement by synaptic dopamine. Using the dopamine/binding ratio of 44:1 and the mean binding difference between patients and controls of 6.8% (22.3% − 15.5% = 6.8%), there is a difference in synaptic dopamine concentrations between patients and controls of ≈300% of baseline (6.8% × 44 = 299.2%). The most conservative interpretation of the data (based on the monkey with the smallest dopamine pulse) resulted in a dopamine/binding ratio that was still quite substantial. Future studies will be needed to further clarify the relationship between dopamine release and striatal radiotracer binding levels.
Previous reports have provided important validation for this brain imaging method. Studies in rodents (17–19, 33), nonhuman primates (6, 7, 9, 25, 34), and man (10, 11) have demonstrated that endogenous dopamine competes with dopamine receptor radiotracers for dopamine receptor occupancy, a crucial element of in vivo brain imaging methods for estimating dopamine outflow. Raclopride has distinct advantages for deriving estimates of synaptic dopamine levels because it is highly selective and has relatively low affinity for D2 and D3 receptors, so that extracellular dopamine avidly competes with raclopride for binding (12–18). Innis et al. (6) have shown in primates that amphetamine decreased binding of 123I-IBZM in a SPECT study, an effect that was prevented by dopamine depletion by reserpine. Dewey et al. (7) reported that in nonhuman primates, striatal [11C]raclopride total binding was significantly decreased (percent binding change) by amphetamine (16.2%), the potent dopamine uptake inhibitor GBR-12909 (22.1%), and the biogenic amine-depleting agent tetrabenazine (28.3%). Laruelle et al. (11), using IBZM/SPECT, found that amphetamine (0.3 mg/kg) causes a 15% decrease in striatal D2 binding in eight healthy human subjects, data that resembles changes observed in our healthy controls. Psychostimulants decrease blood flow in the striatum so that radiotracer reduction there is not secondary to washout by enhanced blood flow (12). It has also been shown that increasing age is related to decreases in psychostimulant-induced synaptic dopamine concentrations (10), a variable controlled for in the present study.
Baseline (preamphetamine administration) striatal binding ratios were not significantly different between controls and schizophrenic patients. These data are similar to several previous PET and SPECT studies reporting no differences in striatal D2 receptor densities between controls and schizophrenic patients (8, 35–39), and are in contrast to work with spiperone radiotracers (40, 41), which showed elevated D2 levels in schizophrenic patients. Note that our specific binding index measures free receptor concentrations, so we cannot exclude the possibility that there are group differences in resting dopamine levels and/or total D2 levels.
The behavioral response to amphetamine observed in this study was modest. No control and only one schizophrenic patient experienced an exacerbation in psychosis. Studies in healthy populations indicate that chronic amphetamine use, as opposed to a single dose, is more consistently associated with induction of psychosis (4). In addition, a relatively low amphetamine dose (0.2 mg/kg) was selected for safety and ethical reasons, which may have contributed to modest psychotogenic responses. We found a significant correlation for amphetamine-induced changes in BPRS total scores, a measure of global symptomatology, and binding changes in the schizophrenic patients, suggesting that these clinical changes are related to the magnitude of released dopamine. As noted in the scatter plot (Fig. 4), some patients had a mild increase in global symptomatology while others had a mild decrease. This heterogenous behavioral amphetamine response is well documented in the clinical literature (5). We failed to observe a significant relationship between changes in binding and changes in the psychosis subfactor, which might be explained by the limited range in amphetamine-related behavioral change scores.
A hypothesis that could account for enhanced amphetamine-induced dopamine outflow in schizophrenia is an alteration in the distribution of intracellular dopamine. Intracellular dopamine exists in free cytoplasmic pools and bound vesicular pools (42). Amphetamine, particularly in low doses, is thought to selectively release cytoplasmic dopamine through a process of Ca2+-independent accelerative exchange-diffusion involving expression of dopamine into the extracellular space via the transporter (43). Thus, schizophrenia may be associated with greater free-to-bound intracellular dopamine ratios than controls. Because cytoplasmic dopamine is derived from newly synthesized dopamine prior to vesicularization and dopamine retrieved from the extracellular space, mechanisms involved in dopamine synthesis, vesicular formation, and uptake are reasonable candidates to explain enhanced dopamine release in this illness. Other mechanisms by which amphetamine increases extracellular dopamine include inhibition of monoamine oxidase (44, 45) and uptake blockade (46), but these mechanisms appear to play a physiologic role at only very high amphetamine doses (47) and may not be relevant to this study.
In summary, we tested the hypothesis proposed more than two decades ago (1, 3) that schizophrenia is associated with increased amphetamine-induced synaptic dopamine concentrations by employing a relatively novel in vivo brain imaging technique. We found that schizophrenic patients had greater reductions in amphetamine-related [11C]raclopride striatal binding ratios than controls, which reflected larger increases in synaptic dopamine levels. Inferences drawn from a study of the simultaneous examination of amphetamine-induced changes in striatal extracellular dopamine levels and [11C]raclopride binding ratios in nonhuman primates suggest that binding differences between patients and controls reflect substantial differences in dopamine levels. This experiment provides direct evidence for the hypothesis of elevated psychostimulant-induced synaptic dopamine concentrations in this illness.
Acknowledgments
We thank the National Institutes of Health Clinical Center PET Department staff, C. J. Endres, D. Pinsky, I. Elman, and the 4-East Clinical staff.
ABBREVIATIONS
- SPECT
single photon emission tomography
- PET
positron-emission tomography
- BPRS
Brief Psychiatric Rating Scale
- CSF
cerebrospinal fluid
- 123I-IBZM
iodobenzamide
References
- 1.Van Rossum J M. In: Proceedings Fifth Collegium Internationale Neuropsychopharmacologicum. Brill H, Cole J O, Deniker P, Hippius H, Bradely P B, editors. Amsterdam: North–Holland; 1967. pp. 321–329. [Google Scholar]
- 2.Seeman P, Bzowej N, Guan H, Bergeron C, Reynolds G, Bird E, Riederer P, Jellinger K, Tourtellotte W. Neuropsychopharmacology. 1987;1:5–15. doi: 10.1016/0893-133x(87)90004-2. [DOI] [PubMed] [Google Scholar]
- 3.Matthysse S. Fed Proc. 1973;32:200–205. [PubMed] [Google Scholar]
- 4.Angrist B. In: Amphetamine Psychosis: Clinical Variations of the Syndrome. Cho A, Segal D, editors. San Diego: Academic; 1994. pp. 387–414. [Google Scholar]
- 5.Lieberman J, Kane J, Alvir J. Psychopharmacology (Berlin) 1987;91:415–433. doi: 10.1007/BF00216006. [DOI] [PubMed] [Google Scholar]
- 6.Innis R, Malison R, Al-Tikriti M, Hoffer P, Sybirska E, Seibyl J, Zoghbi S, Baldwin R, Laruelle M, Smith E, Charney D, Heninger G, Elsworth J, Roth R. Synapse. 1992;10:177–184. doi: 10.1002/syn.890100302. [DOI] [PubMed] [Google Scholar]
- 7.Dewey S, Smith G, Logan J, Brodie J, Fowler J, Wolf A. Synapse. 1993;13:350–356. doi: 10.1002/syn.890130407. [DOI] [PubMed] [Google Scholar]
- 8.Laruelle M, Abi-Dargham A, van Dyck H, Gill R, D’Souza D, Erdos J, McCance-Katz E, Rosenblatt W, Fingado C, Zoghbi S, Baldwin R, Seibyl J P, Krystal J, Charney D, Innis R. Proc Natl Acad Sci USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewey S, Smith G, Logan J, Brodie J, Yu D, Ferrieri R, King P, MacGregor R, Martin T, Wolf A, Volkow N, Fowler J, Meller E. J Neurosci. 1992;12:3773–3780. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkow N, Wang G-J, Fowler J, Logan J, Schlyer D, Hitzemann R, Lieberman J, Angrist B, Pappas N, MacGregor R, Burr G, Cooper T, Wolf A. Synapse. 1994;16:255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- 11.Laruelle M, Abi-Dargham A, van Dyck C, Rosenblatt W, Zea-Ponce Y, Zoghbi S, Baldwin R, Charney D, Hoffer P, Kung H, Innis R. J Nucl Med. 1995;36:1182–1190. [PubMed] [Google Scholar]
- 12.Wang G-J, Volkow N, Fowler J, Ferrieri R, Schlyer D, Axeloff D, Pappas N, Lieberman J, King P, Warner D, Wong C, Hitzemann R, Wolf A. Life Sci. 1994;54:143–146. doi: 10.1016/0024-3205(94)00873-6. [DOI] [PubMed] [Google Scholar]
- 13.Farde L, Ehrin E, Eriksson L, Greitz T, Hall H, Hedstrom C-G, Litton J, Sedvall G. Proc Natl Acad Sci USA. 1985;82:8863–8867. doi: 10.1073/pnas.82.11.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farde L, Hall H, Ehrin E, Sedvall G. Science. 1986;231:258–260. doi: 10.1126/science.2867601. [DOI] [PubMed] [Google Scholar]
- 15.Seeman P, Grigoriadis D, Niznik H. Drug Dev Res. 1986;9:63–69. [Google Scholar]
- 16.Seeman P, Guan H, Niznik H. Synapse. 1989;3:96–97. doi: 10.1002/syn.890030113. [DOI] [PubMed] [Google Scholar]
- 17.Ross S, Jackson D. Naunyn-Schmiedeberg’s Arch Pharmacol. 1989;340:6–12. doi: 10.1007/BF00169199. [DOI] [PubMed] [Google Scholar]
- 18.Young T, Wong D, Goldman S, Minkin E, Chen C, Matsumara K, Scheffel U, Wagner H. Synapse. 1991;7:188–194. doi: 10.1002/syn.890090305. [DOI] [PubMed] [Google Scholar]
- 19.Hume S, Myers R, Bloomfield P, Opacka-Juffry J, Cremer J, Ahier R, Luthra S, Brooks D, Lammertsma A. Synapse. 1992;12:47–54. doi: 10.1002/syn.890120106. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Skirboll S, Aigner T, Saunders R, Hsiao J, Bankiewicz K. Exp Neurol. 1990;11:181–186. doi: 10.1016/0014-4886(90)90028-q. [DOI] [PubMed] [Google Scholar]
- 21.Saunders R, Kolachana B, Weinberger D. Exp Brain Res. 1994;98:44–52. doi: 10.1007/BF00229108. [DOI] [PubMed] [Google Scholar]
- 22.Hsiao J, Ball B, Morrison P, Mefford I, Bungay P. J Neurochem. 1990;154:1449–1452. doi: 10.1111/j.1471-4159.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 23.Saunders R, Aigner T, Frank J. Exp Brain Res. 1990;81:443–446. doi: 10.1007/BF00228139. [DOI] [PubMed] [Google Scholar]
- 24.Kolachana B, Saunders R, Weinberger D. J Neurosci Methods. 1994;55:1–6. doi: 10.1016/0165-0270(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 25.Carson, R., Breier, A., deBartolomeis, A., Saunders, R., Su, T., Schmall, B., Der, M. G., Pickar, D. & Eckelman, W. C. (1996) J. Cereb. Blood Flow Metab., in press. [DOI] [PubMed]
- 26.Carson R, Channing M A, Blasberg R G, Dunn B B, Cohen R M, Rice K C, Herscovitch P. J Cereb Blood Flow Metab. 1993;13:24–42. doi: 10.1038/jcbfm.1993.6. [DOI] [PubMed] [Google Scholar]
- 27.Spitzer, R., Williams, J., Gibbon, M. & First, M. Structured Clinical Interview for DSM-III-R: Patient Edition (SCID-P, Version 1.0) (American Psychiatric Press, Washington, DC).
- 28.Overall J, Gorham D. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 29.Endres C, Carson R, Kolachana B, Su T, Saunders R, Eckelman W, Breier A. J Nucl Med. 1996;37:10. (abstr.). [Google Scholar]
- 30.Segal D, Kuczenski R. Brain Res. 1992;571:330–337. doi: 10.1016/0006-8993(92)90672-v. [DOI] [PubMed] [Google Scholar]
- 31.Cadoni C, Pinna A, Russi G, Consolo S, Di Chiara G. Neuroscience. 1995;65:1027–1039. doi: 10.1016/0306-4522(94)00507-2. [DOI] [PubMed] [Google Scholar]
- 32.Moghaddam B, Berridge C, Goldman-Rakic P, Bunney B, Roth R. Synapse. 1993;13:215–222. doi: 10.1002/syn.890130304. [DOI] [PubMed] [Google Scholar]
- 33.Ross S. J Neurochem. 1991;56:22–29. doi: 10.1111/j.1471-4159.1991.tb02557.x. [DOI] [PubMed] [Google Scholar]
- 34.Kessler R, Votaw J, de Paulis T, Bingham D, Ansari M, Mason N, Holburn G, Schmidt D, Votaw D, Manning R. Synapse. 1993;15:169–176. doi: 10.1002/syn.890150302. [DOI] [PubMed] [Google Scholar]
- 35.Farde L, Wiesel F, Stone-Elander S, Halldin C, Nordstrom A L, Hall H, Sedvall G. Arch Gen Psychiatry. 1990;47:213–219. doi: 10.1001/archpsyc.1990.01810150013003. [DOI] [PubMed] [Google Scholar]
- 36.Martinot J I, Paillere-Martinot M L, Loc’h C, Hardy P, Poirier M F, Mazoyer B, Beaufils B, Maziefre B, Alliaire J F, Syrota A. Br J Psychiatry. 1991;158:346–350. doi: 10.1192/bjp.158.3.346. [DOI] [PubMed] [Google Scholar]
- 37.Hietala J, Syvalahti E, Vuorio K, Nagren K, Lehikoinen P, Ruotsalainen U, Raakkolainen V, Lehtinen V, Wegelius U. Arch Gen Psychiatry. 1994;51:116–123. doi: 10.1001/archpsyc.1994.03950020040004. [DOI] [PubMed] [Google Scholar]
- 38.Pilowsky L S, Costa D C, Ell P J, Verhoeff N P L G, Murray R M, Kerwin R W. Br J Psychiatry. 1994;164:16–26. doi: 10.1192/bjp.164.1.16. [DOI] [PubMed] [Google Scholar]
- 39.Nordstrom A L, Farde L, Eriksson L, Halldin C. Psychiatry Res. 1995;61:67–83. doi: 10.1016/0925-4927(95)02732-d. [DOI] [PubMed] [Google Scholar]
- 40.Wong D F, Wagner H N, Tune L E, Dannals R F, Pearlson G D, Links J M, Tamminga C A, Broussolle E P, Ravert H T, Wilson A A, Toung J K, Malat J, Williams J A, O’Tuama L A, Snyder S H, Kuhar M J, Gjedde A. Science. 1986;234:1558–1563. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]
- 41.Tune L E, Wong D F, Pearlson G, Strauss M, Young T, Shaya E K, Dannals R F, Wilson A A, Ravert H T, Sapp J, Cooper T, Chase G A, Wagner H N. Psychiatry Res. 1994;49:219–237. doi: 10.1016/0165-1781(93)90063-m. [DOI] [PubMed] [Google Scholar]
- 42.McMillen B, German D, Shore P. Biochem Pharmacol. 1980;29:3045–3050. doi: 10.1016/0006-2952(80)90444-x. [DOI] [PubMed] [Google Scholar]
- 43.Fischer J, Cho A. J Pharmacol Exp Ther. 1979;192:642–653. [Google Scholar]
- 44.Green A, El Hait A. Biochem Pharmacol. 1980;29:2781–2789. doi: 10.1016/0006-2952(80)90012-x. [DOI] [PubMed] [Google Scholar]
- 45.Miller H, Shore P, Clarke D. Biochem Pharmacol. 1980;29:1347–1354. doi: 10.1016/0006-2952(80)90429-3. [DOI] [PubMed] [Google Scholar]
- 46.Ross S, Renyi A. Acta Pharmacol Toxicol. 1964;21:226–239. doi: 10.1111/j.1600-0773.1964.tb01787.x. [DOI] [PubMed] [Google Scholar]
- 47.Kuczenski R, Segal D. In: Neurochemistry of Amphetamine. Cho A, Segal D, editors. San Diego: Academic; 1994. pp. 81–113. [Google Scholar]