Abstract
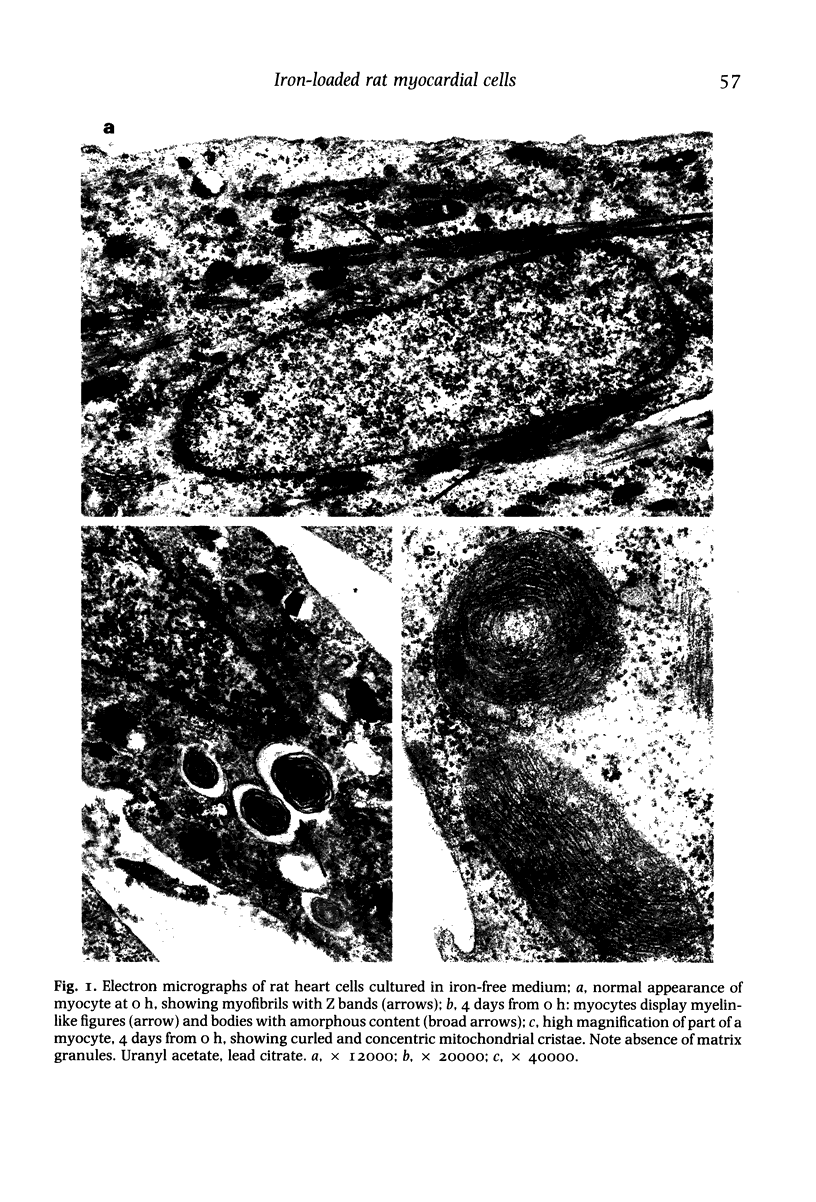
The pathological changes induced by in-vitro iron-loading or cultured rat myocardial cells were studied. Cells were exposed to 59Fe-labelled ferric ammonium citrate for up to 24 h followed by 24-72 h chase experiment. After 24 h exposure 29% of the total cellular radioactivity was found in ferritin, 10% in non-ferritin heat supernatant and 61% in an insoluble heat-precipitable form. Mössbauer spectroscopy showed a gradual shift from intracellular iron particles less than 1.8 nm in diameter, through particles of intermediate size, to ferritin-like aggregates over 3.0 nm in diameter, reaching about 20% of total iron by 24 h. Ultrastructural studies showed premature damage such as mitochondrial abnormalities and excessive autophagocytosis. Small, 2.0-5.0 nm electron-dense cytosolic particles were noticed at 3 h of iron loading and reached maximal concentrations at 6 h. This was followed by accumulation of the small particles and of typical iron-rich ferritin cores within siderosomes. Because of the limited duration of iron loading and the high concentrations of non-transferrin inorganic iron employed, the present model is more relevant to acute than chronic iron overload. The efficient incorporation of large amounts of iron within ferritin molecules and its subsequent segregation, together with other smaller particles, within membrane-bound bodies, may represent a defence mechanism limiting iron toxicity in the face of advanced cytosiderosis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buja L. M., Roberts W. C. Iron in the heart. Etiology and clinical significance. Am J Med. 1971 Aug;51(2):209–221. doi: 10.1016/0002-9343(71)90240-3. [DOI] [PubMed] [Google Scholar]
- Hernández-Yago J., Knecht E., Martínez-Ramón A., Grisolía S. Autophagy of ferritin incorporated into the cytosol of Hela cells by liposomes. Cell Tissue Res. 1980;205(2):303–309. doi: 10.1007/BF00234688. [DOI] [PubMed] [Google Scholar]
- Iancu T. C., Neustein H. B. Ferritin in human liver cells of homozygous beta-thalassaemia: ultrastructural observations. Br J Haematol. 1977 Dec;37(4):527–535. doi: 10.1111/j.1365-2141.1977.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Iancu T. C., Rabinowitz H., Brissot P., Guillouzo A., Deugnier Y., Bourel M. Iron overload of the liver in the baboon. An ultrastructural study. J Hepatol. 1985;1(3):261–275. doi: 10.1016/s0168-8278(85)80054-4. [DOI] [PubMed] [Google Scholar]
- Jacobs A., Hoy T., Humphrys J., Perera P. Iron overload in Chang cell cultures: biochemical and morphological studies. Br J Exp Pathol. 1978 Oct;59(5):489–498. [PMC free article] [PubMed] [Google Scholar]
- Link G., Pinson A., Hershko C. Heart cells in culture: a model of myocardial iron overload and chelation. J Lab Clin Med. 1985 Aug;106(2):147–153. [PubMed] [Google Scholar]
- Schwartz P., Piper H. M., Spahr R., Spieckermann P. G. Ultrastructure of cultured adult myocardial cells during anoxia and reoxygenation. Am J Pathol. 1984 Jun;115(3):349–361. [PMC free article] [PubMed] [Google Scholar]
- Torrance J. D., Bothwell T. H. A simple technique for measuring storage iron concentrations in formalinised liver samples. S Afr J Med Sci. 1968 Apr;33(1):9–11. [PubMed] [Google Scholar]
- White G. P., Jacobs A. Iron uptake by Chang cells from transferrin, nitriloacetate and citrate complexes: the effects of iron-loading and chelation with desferrioxamine. Biochim Biophys Acta. 1978 Oct 3;543(2):217–225. doi: 10.1016/0304-4165(78)90066-1. [DOI] [PubMed] [Google Scholar]
- Yagev S., Heller M., Pinson A. Changes in cytoplasmic and lysosomal enzyme activities in cultured rat heart cells: the relationship to cell differentiation and cell population in culture. In Vitro. 1984 Dec;20(12):893–898. doi: 10.1007/BF02619662. [DOI] [PubMed] [Google Scholar]