Abstract
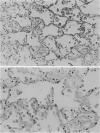
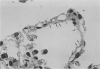
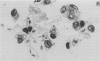
Pneumocystis carinii pneumonia (PCP) was provoked in the ferret, Mustela pulorius furo, by immunosuppression with daily long-term administration of cortisone acetate, 10-20 mg/kg subcutaneously for 9 to 10 weeks, Microscopically P. carinii was observed in the lungs of all 11 treated animals: mild to moderate in five and extensive disease in six. The histopathological features of PCP in the ferret included interstitial pneumonitis, scant mononuclear cell alveolitis, with abundant cysts and trophozoites visible in a focal distribution. There were few neutrophils present. Electron microscopy showed large numbers of both cysts and trophozoites in close association with type I cells. No bacterial pathogens were isolated from the lungs of immunosuppressed animals but an unexplained eosinophilic enteritis was present in treated animals. P carinii pneumonia developed without significant body weight loss during corticosteroid administration, unlike previously described studies using corticosteroid-treated rodents. Ferrets thus appear to be a 'steroid resistant' animal, like man, and therefore a more suitable model for immunological studies of host response to PCP than rodents. This new model also has practical advantages over previously described animal models of PCP, including larger lung and airway size.
Full text
PDF

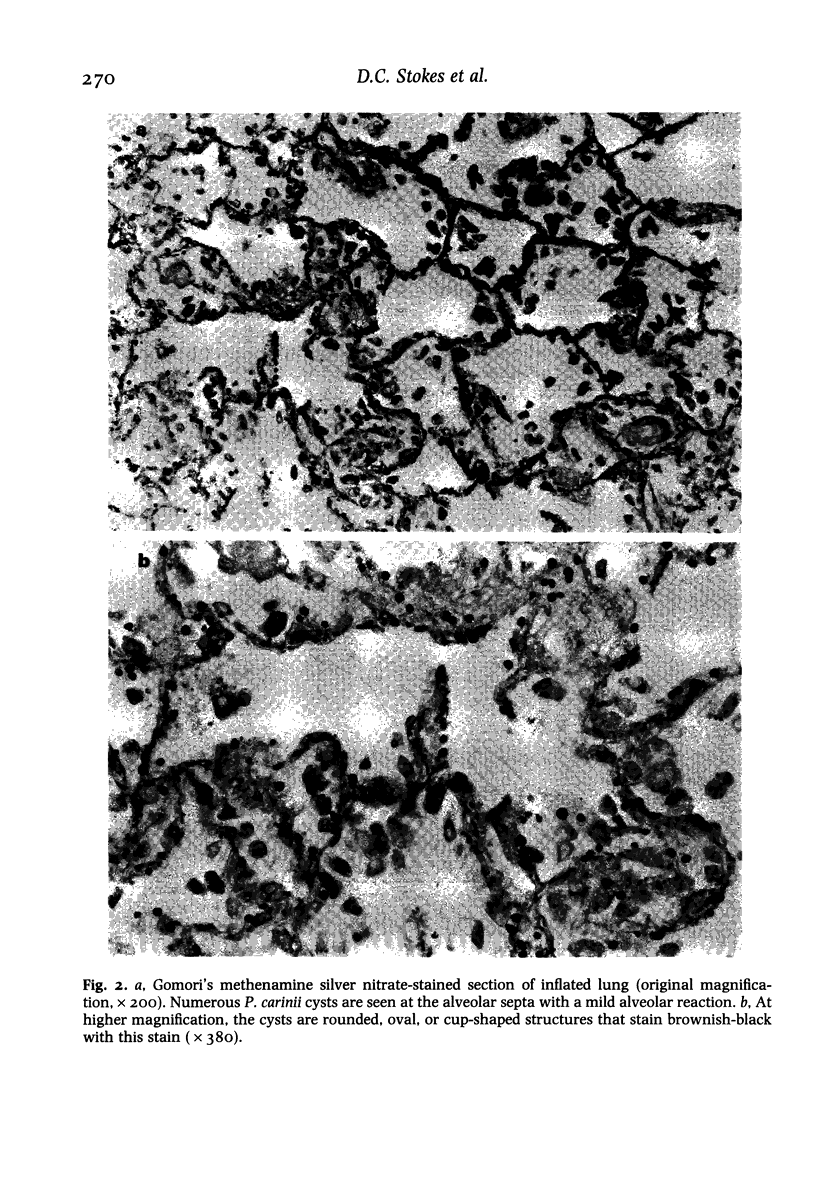
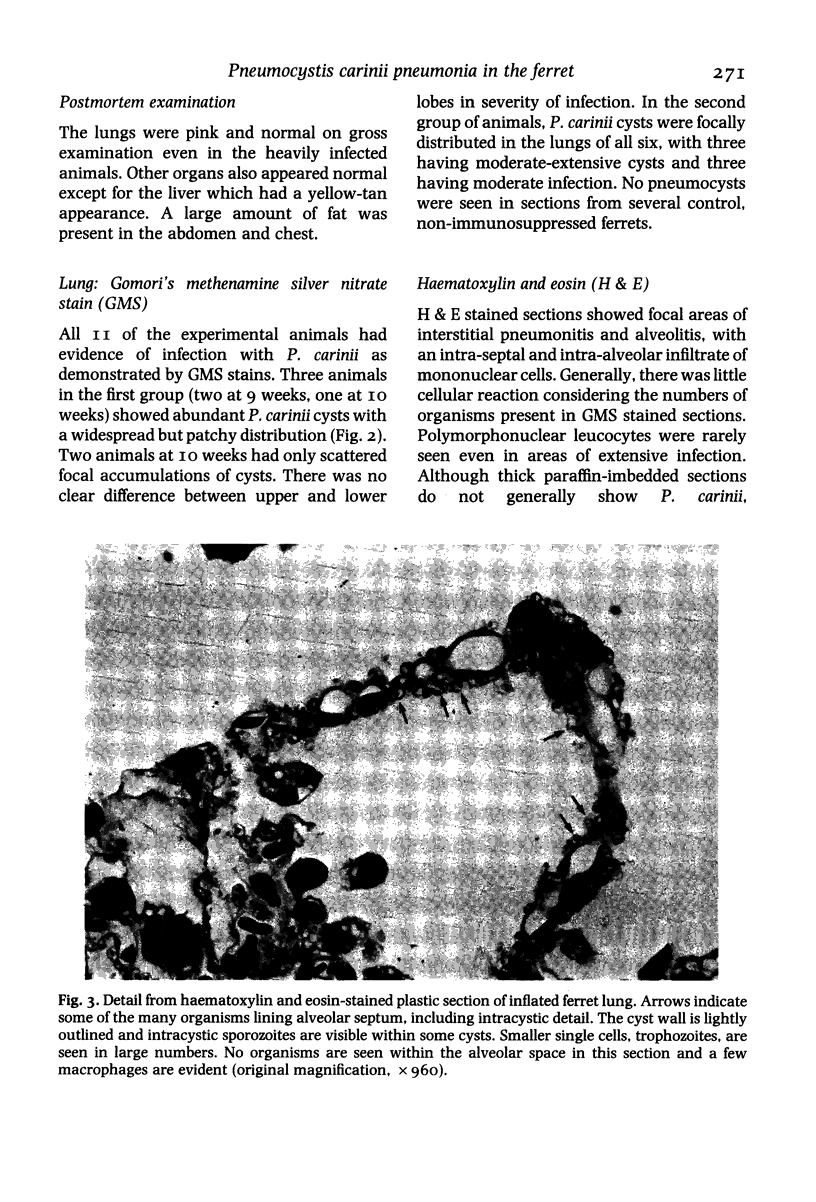
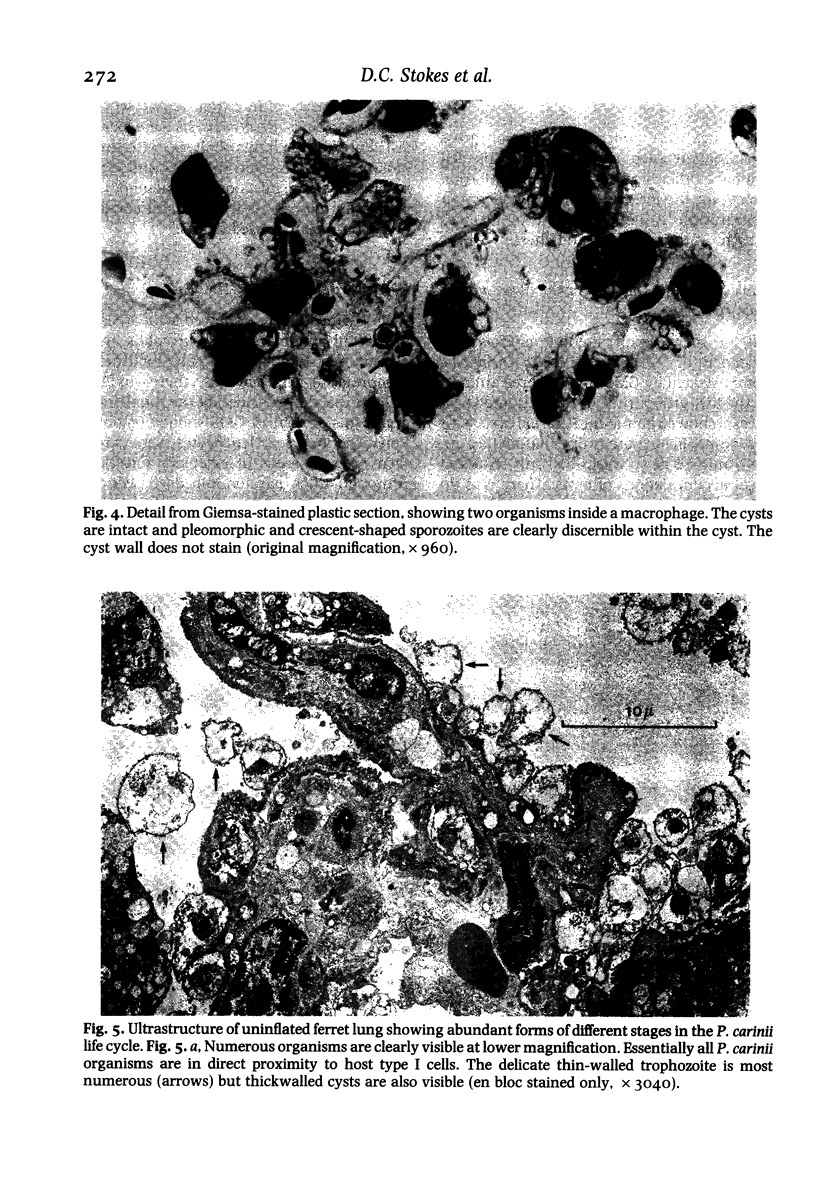
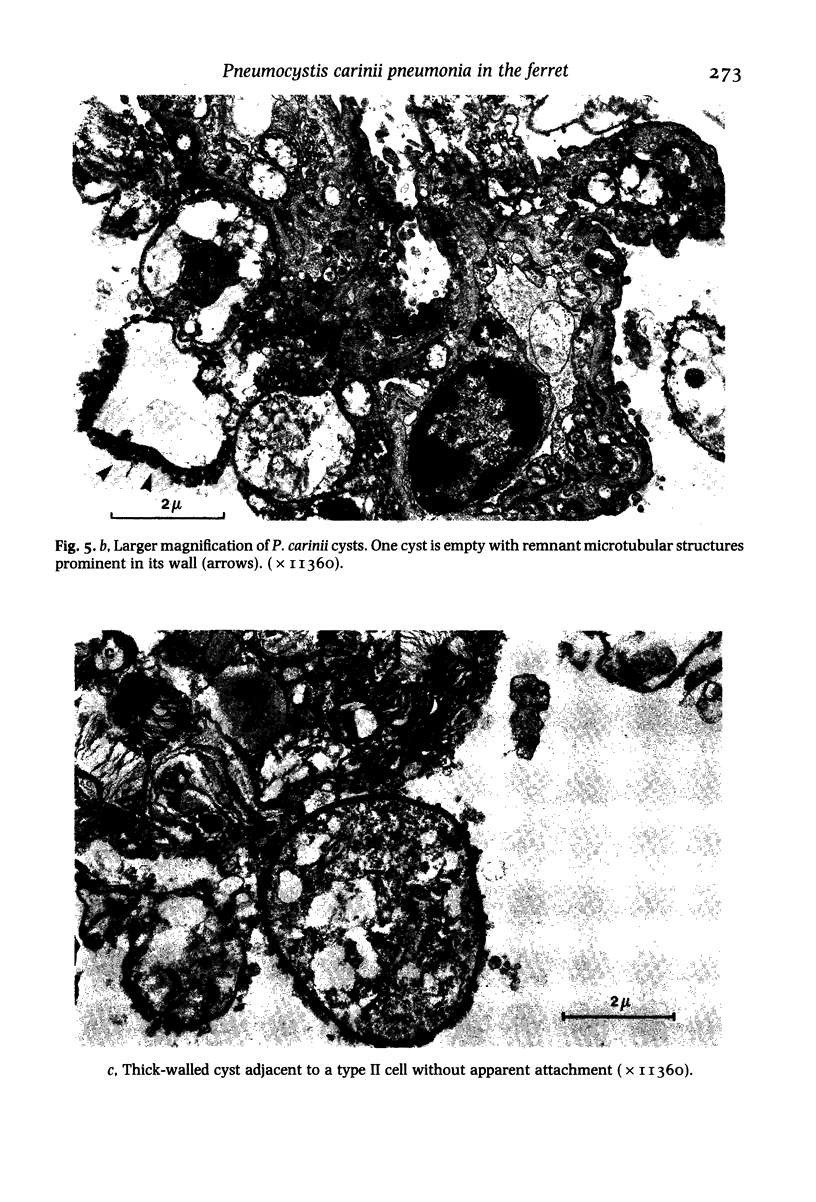



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco P., Ponzi A., Bonucci E. Basic and 'special' stains for plastic sections in bone marrow histopathology, with special reference to May-Grünwald Giemsa and enzyme histochemistry. Basic Appl Histochem. 1984;28(3):265–279. [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Frenkel J. K., Good J. T., Shultz J. A. Latent Pneumocystis infection of rats, relapse, and chemotherapy. Lab Invest. 1966 Oct;15(10):1559–1577. [PubMed] [Google Scholar]
- Gigliotti F., Stokes D. C., Cheatham A. B., Davis D. S., Hughes W. T. Development of murine monoclonal antibodies to Pneumocystis carinii. J Infect Dis. 1986 Aug;154(2):315–322. doi: 10.1093/infdis/154.2.315. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. S., Schroff R., Schanker H. M., Weisman J. D., Fan P. T., Wolf R. A., Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981 Dec 10;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Hughes W. T., McNabb P. C., Makres T. D., Feldman S. Efficacy of trimethoprim and sulfamethoxazole in the prevention and treatment of Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1974 Mar;5(3):289–293. doi: 10.1128/aac.5.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. T. Natural mode of acquisition for de novo infection with Pneumocystis carinii. J Infect Dis. 1982 Jun;145(6):842–848. doi: 10.1093/infdis/145.6.842. [DOI] [PubMed] [Google Scholar]
- Hughes W. T., Price R. A., Sisko F., Havron W. S., Kafatos A. G., Schonland M., Smythe P. M. Protein-calorie malnutrition. A host determinant for Pneumocystis carinii infection. Am J Dis Child. 1974 Jul;128(1):44–52. doi: 10.1001/archpedi.1974.02110260046008. [DOI] [PubMed] [Google Scholar]
- Hughes W. T., Smith B. Provocation of infection due to Pneumocystis carinii by cyclosporin A. J Infect Dis. 1982 May;145(5):767–767. doi: 10.1093/infdis/145.2.767. [DOI] [PubMed] [Google Scholar]
- LONG D. A., SHEWELL J. A species difference with regard to the effect of cortisone acetate on body weight, gamma-globulin and circulating antitoxin levels. J Hyg (Lond) 1956 Dec;54(4):452–460. doi: 10.1017/s0022172400044739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes D. C., Hughes W. T., Alderson P. O., King R. E., Garfinkel D. J. Lung mechanics, radiography and 67Ga scintigraphy in experimental Pneumocystis carinii pneumonia. Br J Exp Pathol. 1986 Jun;67(3):383–393. [PMC free article] [PubMed] [Google Scholar]
- Vinegar A., Sinnett E. E., Kosch P. C. Respiratory mechanics of a small carnivore: the ferret. J Appl Physiol Respir Environ Exerc Physiol. 1982 Apr;52(4):832–837. doi: 10.1152/jappl.1982.52.4.832. [DOI] [PubMed] [Google Scholar]
- WELLER R. Zur Erzeugung von Pneumocystosen im Tierversuch. Z Kinderheilkd. 1955;76(4):366–378. [PubMed] [Google Scholar]
- Walzer P. D., Powell R. D., Jr, Yoneda K., Rutledge M. E., Milder J. E. Growth characteristics and pathogenesis of experimental Pneumocystis carinii pneumonia. Infect Immun. 1980 Mar;27(3):928–937. doi: 10.1128/iai.27.3.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Young L. S. Clinical relevance of animal models of Pneumocystis carinii pneumonia. Diagn Microbiol Infect Dis. 1984 Jan;2(1):1–6. doi: 10.1016/0732-8893(84)90016-6. [DOI] [PubMed] [Google Scholar]
- Yoneda K., Walzer P. D. Interaction of Pneumocystis carinii with host lungs: an ultrastructural study. Infect Immun. 1980 Aug;29(2):692–703. doi: 10.1128/iai.29.2.692-703.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]