Abstract
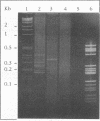
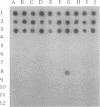
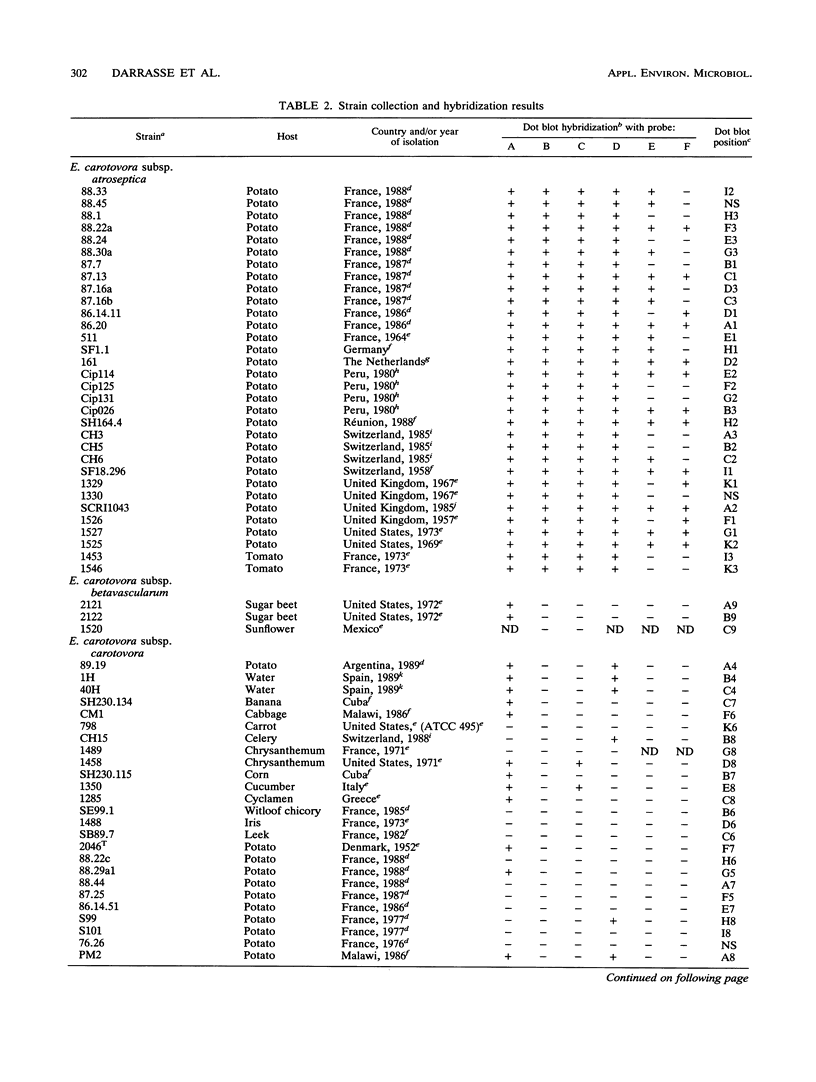
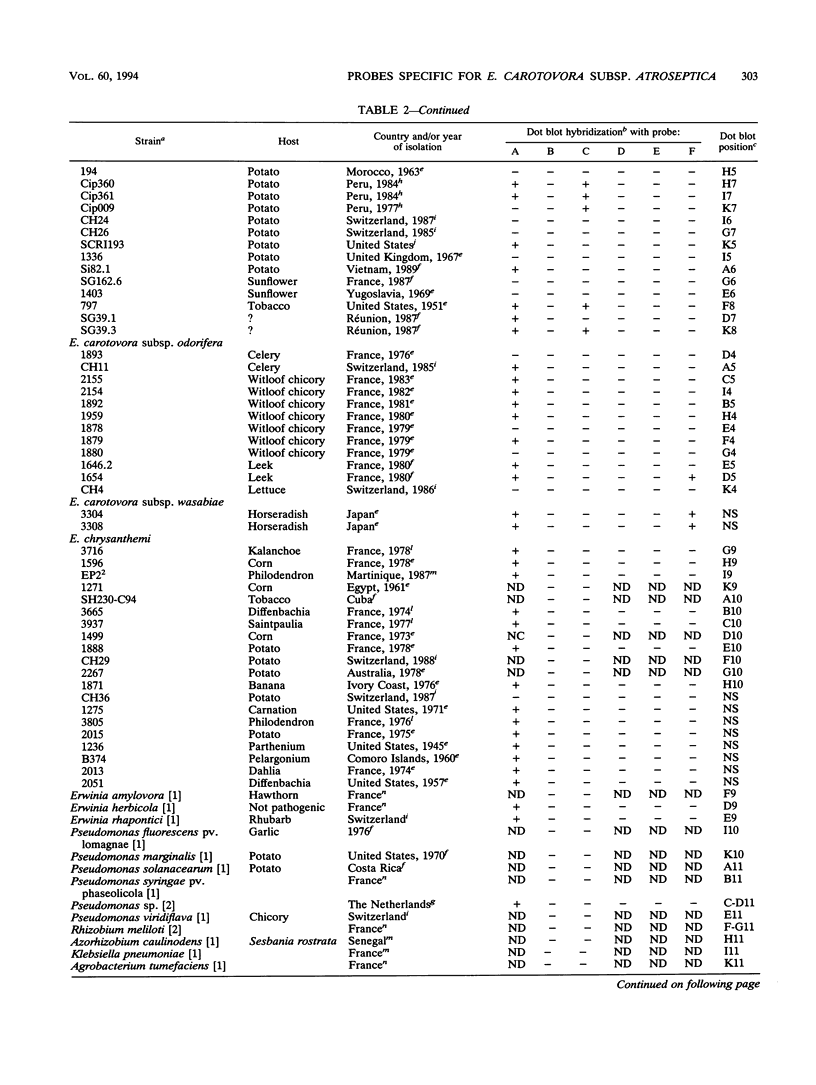
Erwinia carotovora subsp. atroseptica is a pathogen of potatoes in Europe because of its ability to induce blackleg symptoms early in the growing season. However, E. carotovora subsp. carotovora is not able to produce such severe symptoms under the same conditions. On the basis of the technique described by Straus and Ausubel (Proc. Natl. Acad. Sci. USA 87:1889-1893, 1990), we isolated DNA sequences of E. carotovora subsp. atroseptica 86.20 that were absent from the genomic DNA of E. carotovora subsp. carotovora CH26. Six DNA fragments ranging from ca. 180 to 400 bp were isolated, cloned, and sequenced. Each fragment was further hybridized with 130 microorganisms including 87 E. carotovora strains. One probe was specific for typical E. carotovora subsp. atroseptica strains, two probes hybridized with all E. carotovora subsp. atroseptica strains and with a few E. carotovora subsp. carotovora strains, and two probes recognized only a subset of E. carotovora subsp. atroseptica strains. The last probe was absent from the genomic DNA of E. carotovora subsp. carotovora CH26 but was present in the genomes of many strains, including those of other species and genera. This probe is homologous to the putP gene of Escherichia coli, which encodes a proline carrier. Further use of the probes is discussed.
Full text
PDF



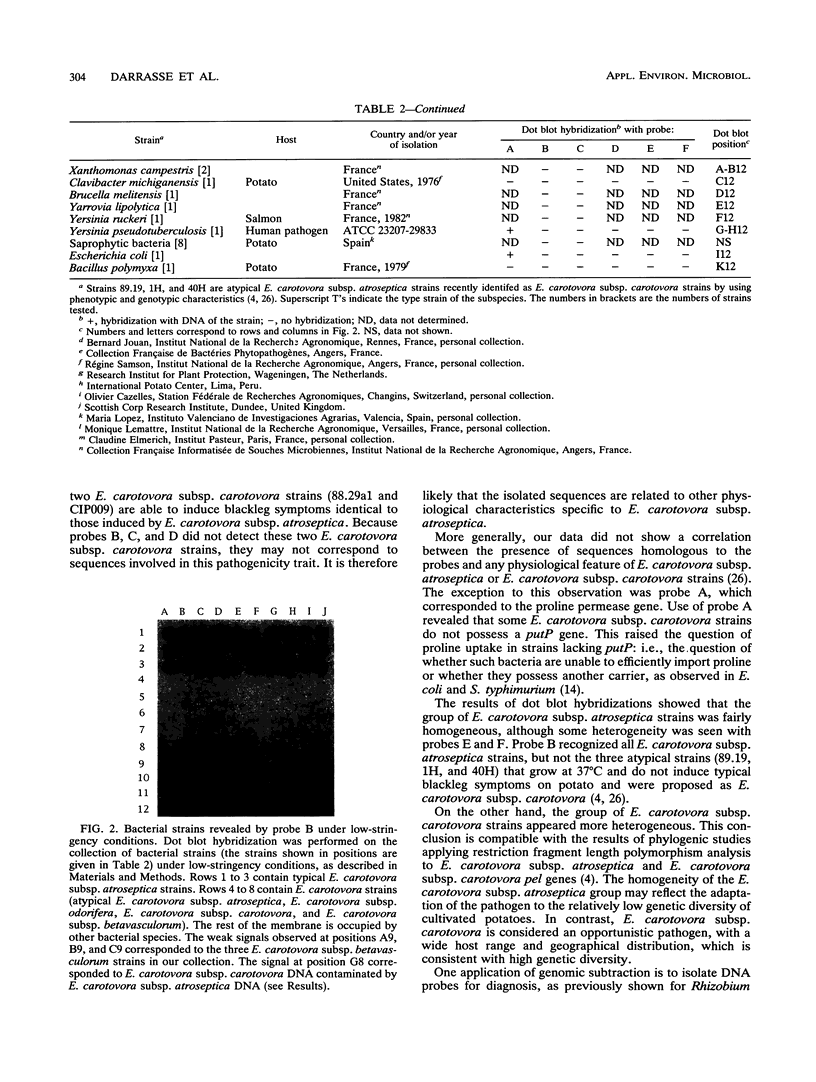


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjourson A. J., Cooper J. E. Isolation of Rhizobium loti Strain-Specific DNA Sequences by Subtraction Hybridization. Appl Environ Microbiol. 1988 Nov;54(11):2852–2855. doi: 10.1128/aem.54.11.2852-2855.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Klotz L. C., Zimm B. H. Size of DNA determined by viscoelastic measurements: results on bacteriophages, Bacillus subtilis and Escherichia coli. J Mol Biol. 1972 Dec 30;72(3):779–800. doi: 10.1016/0022-2836(72)90191-x. [DOI] [PubMed] [Google Scholar]
- Kotoujansky A., Lemattre M., Boistard P. Utilization of a thermosensitive episome bearing transposon TN10 to isolate Hfr donor strains of Erwinia carotovora subsp. chrysanthemi. J Bacteriol. 1982 Apr;150(1):122–131. doi: 10.1128/jb.150.1.122-131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Skorupa E. S., Kemper B. Single stranded DNA SP6 promoter plasmids for engineering mutant RNAs and proteins: synthesis of a 'stretched' preproparathyroid hormone. Nucleic Acids Res. 1985 Feb 25;13(4):1103–1118. doi: 10.1093/nar/13.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K., Selander R. K. Evolutionary genetics of the proline permease gene (putP) and the control region of the proline utilization operon in populations of Salmonella and Escherichia coli. J Bacteriol. 1992 Nov;174(21):6886–6895. doi: 10.1128/jb.174.21.6886-6895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. Polymerase chain reaction: applications in environmental microbiology. Annu Rev Microbiol. 1991;45:137–161. doi: 10.1146/annurev.mi.45.100191.001033. [DOI] [PubMed] [Google Scholar]
- Straus D., Ausubel F. M. Genomic subtraction for cloning DNA corresponding to deletion mutations. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1889–1893. doi: 10.1073/pnas.87.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Hanafey M. K., Rafalski J. A., Tingey S. V. Genetic analysis using random amplified polymorphic DNA markers. Methods Enzymol. 1993;218:704–740. doi: 10.1016/0076-6879(93)18053-f. [DOI] [PubMed] [Google Scholar]