Abstract
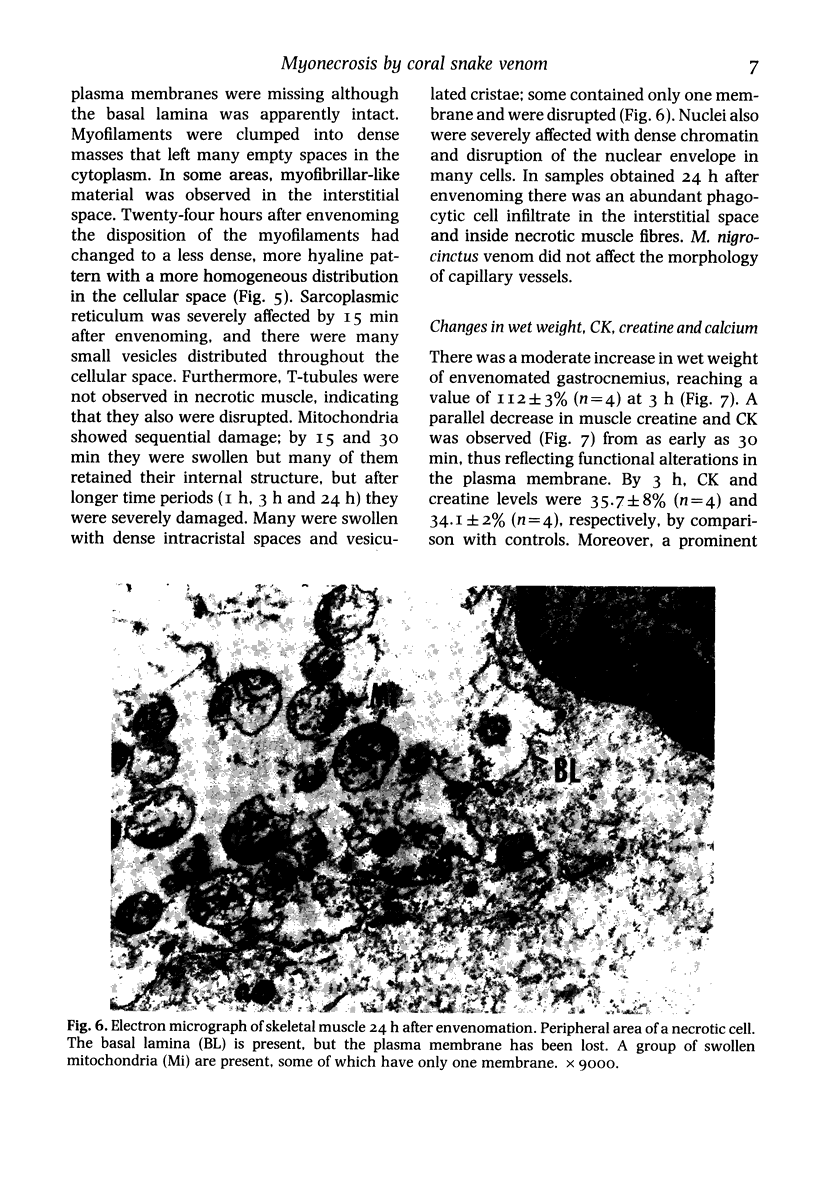
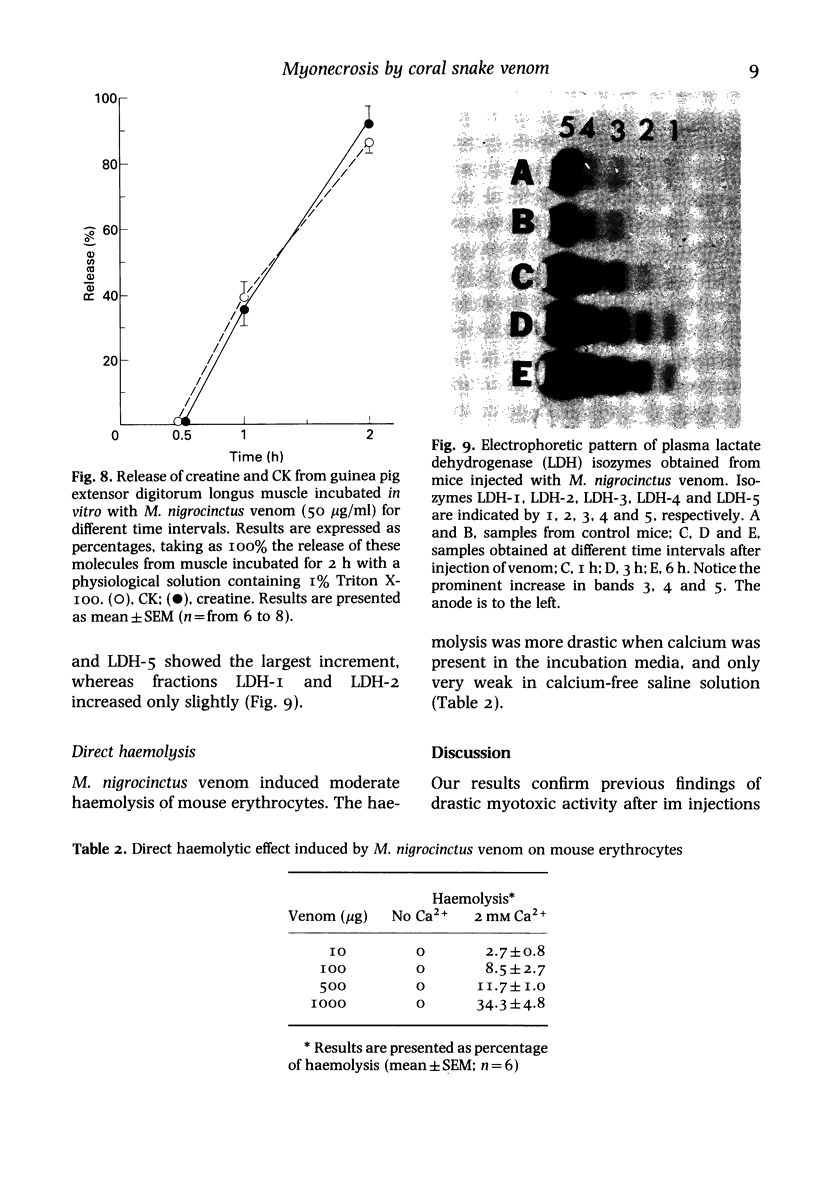
The mode by which coral snake (Micrurus nigrocinctus) venom affects skeletal muscle was studied using a combined approach. The venom induced early functional and structural alterations in the plasma membrane of muscle cells, suggesting that sarcolemma is the primary site of action of this venom. This was shown by the presence of wedge-shaped ('delta') lesions at the periphery of the cells, as well as by focal disruptions in the continuity of plasma membrane as early as 15 min after envenomation. After this initial alteration the rest of the organelles were severely affected. Myofilaments were hypercontracted leaving, as a consequence, areas of overstretched myofibrils as well as empty spaces. Eventually, myofilaments formed dense, clumped masses in which the striated structure was totally lost. At 24 h, myofilaments were still disorganized but they presented a more hyaline and homogeneous appearance. As early as 15 and 30 min mitochondria were swollen; later, by I, 3 and 24 h, they showed further alterations such as the presence of dense intracristal spaces and vesiculated cristae, as well as disruption in the integrity of their membranes. Sarcoplasmic reticulum was dilated and disorganized into many small vesicles randomly distributed throughout the cellular space. Moreover, the venom induced a rapid decrease in muscle levels of creatine and creatine-kinase (CK) and a calcium influx. Since the rates of efflux of creatine and CK were similar, it is suggested that the lesions produced in the membrane are large enough to allow the escape of these two molecules. As corroboration of the severe myotoxic effect, envenomated mice excreted reddish urine containing large quantities of myoglobin. Skeletal muscle cells are more susceptible to the action of the venom than erythrocytes, since coral snake venom induced only a mild direct haemolytic effect in vitro and haemolysis is not a significant effect in vivo. M. nigrocinctus venom induced a drastic increase in plasma levels of lactate dehydrogenase. Isozymes LDH-3, LDH-4, and LDH-5 increased markedly, suggesting that the systemic pathology of coral snake envenoming may be more complex than previously thought.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arroyo O., Gutiérrez J. M. Estudio ultraestructural de la mionecrosis inducida en raton por el veneno de terciopelo (Bothrops asper) de Costa Rica. Toxicon. 1981;19(6):773–782. doi: 10.1016/0041-0101(81)90074-x. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Excell B. J., Patel R., Smith B. Changes in motor end-plates resulting from muscle fibre necrosis and regeneration. A light and electron microscopic study of the effects of the depolarizing fraction (cardiotoxin) of Dendroaspis jamesoni venom. J Neurol Sci. 1974 Apr;21(4):391–417. doi: 10.1016/0022-510x(74)90041-0. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J. M., Lomonte B., Portilla E., Cerdas L., Rojas E. Local effects induced by coral snake venoms: evidence of myonecrosis after experimental inoculations of venoms from five species. Toxicon. 1983;21(6):777–783. doi: 10.1016/0041-0101(83)90066-1. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J. M., Ownby C. L., Odell G. V. Isolation of a myotoxin from Bothrops asper venom: partial characterization and action on skeletal muscle. Toxicon. 1984;22(1):115–128. doi: 10.1016/0041-0101(84)90144-2. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Maltin C. A. Myotoxic activity of the crude venom and the principal neurotoxin, taipoxin, of the Australian taipan, Oxyuranus scutellatus. Br J Pharmacol. 1982 May;76(1):61–75. doi: 10.1111/j.1476-5381.1982.tb09191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. L., Hider R. C., Khader F. Effect of phospholipase A on actions of cobra venom cardiotoxins on erythrocytes and skeletal muscle. Biochim Biophys Acta. 1983 Feb;728(2):215–221. doi: 10.1016/0005-2736(83)90474-1. [DOI] [PubMed] [Google Scholar]
- Ishiura S., Nonaka I., Nakase H., Tada A., Sugita H. Two-step mechanism of myofibrillar protein degradation in acute plasmocid-induced muscle necrosis. Biochim Biophys Acta. 1984 Apr 24;798(3):333–342. doi: 10.1016/0304-4165(84)90107-7. [DOI] [PubMed] [Google Scholar]
- La Mar G. N., Smith K. M., Gersonde K., Sick H., Overkamp M. Proton nuclear nagnetic resonance characterization of heme disorder in monomeric insect hemoglobins. J Biol Chem. 1980 Jan 10;255(1):66–70. [PubMed] [Google Scholar]
- Mokri B., Engel A. G. Duchenne dystrophy: electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fiber. Neurology. 1975 Dec;25(12):1111–1120. doi: 10.1212/wnl.25.12.1111. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Classification of microbial, plant and animal cytolysins based on their membrane-damaging effects of human fibroblasts. Biochim Biophys Acta. 1979 Oct 19;557(1):156–169. doi: 10.1016/0005-2736(79)90098-1. [DOI] [PubMed] [Google Scholar]
- Wrogemann K., Pena S. D. Mitochondrial calcium overload: A general mechanism for cell-necrosis in muscle diseases. Lancet. 1976 Mar 27;1(7961):672–674. doi: 10.1016/s0140-6736(76)92781-1. [DOI] [PubMed] [Google Scholar]