Abstract
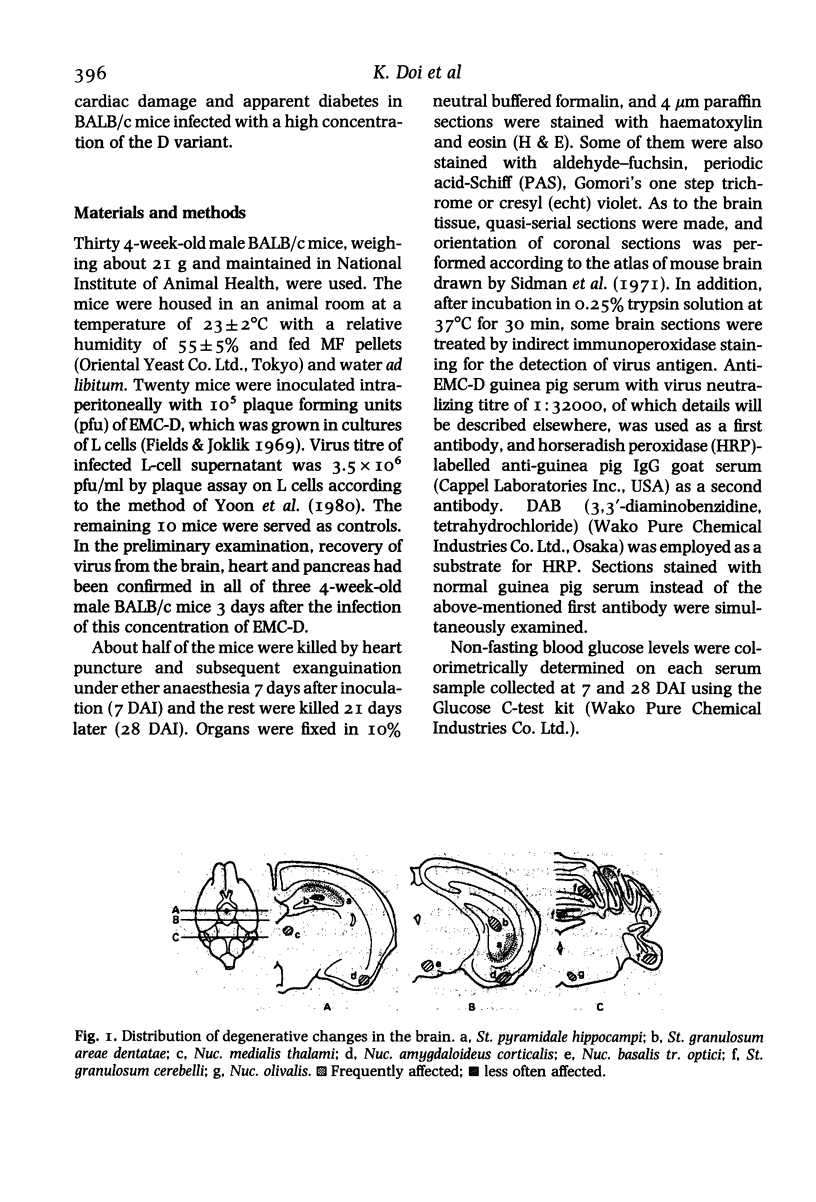
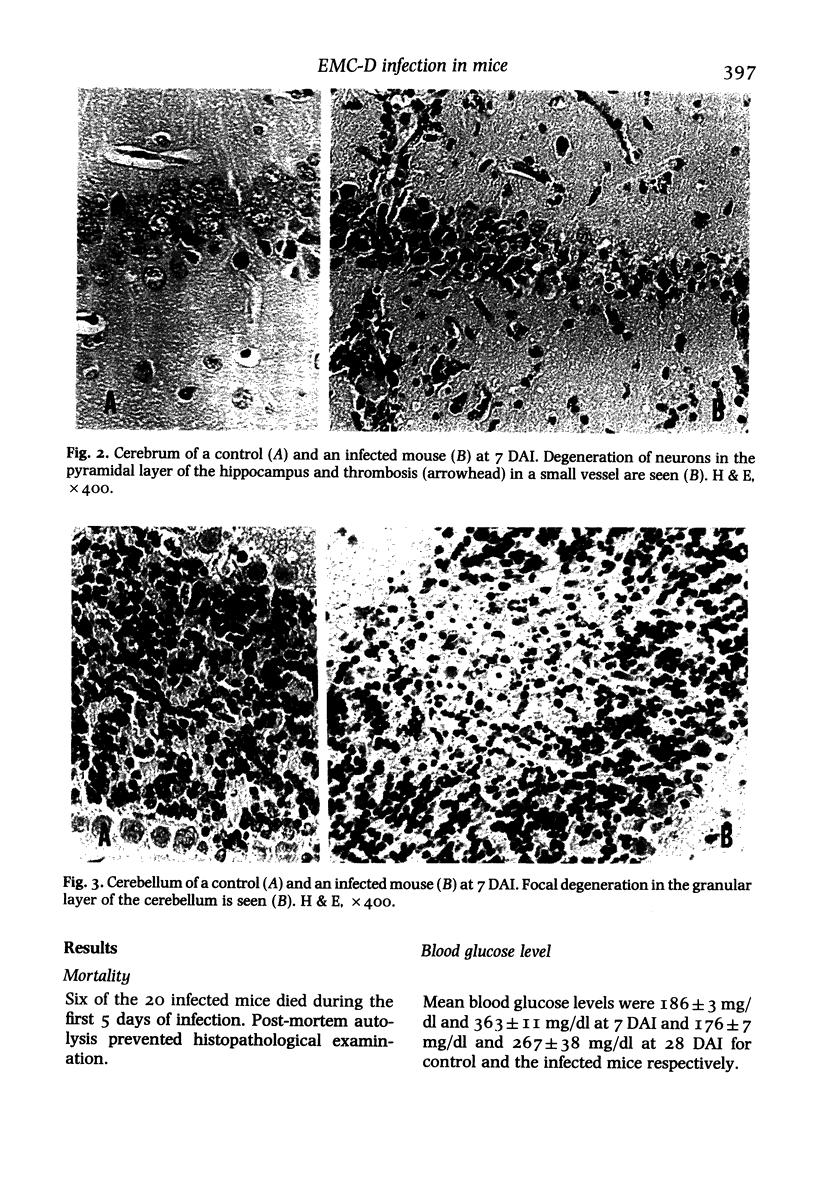
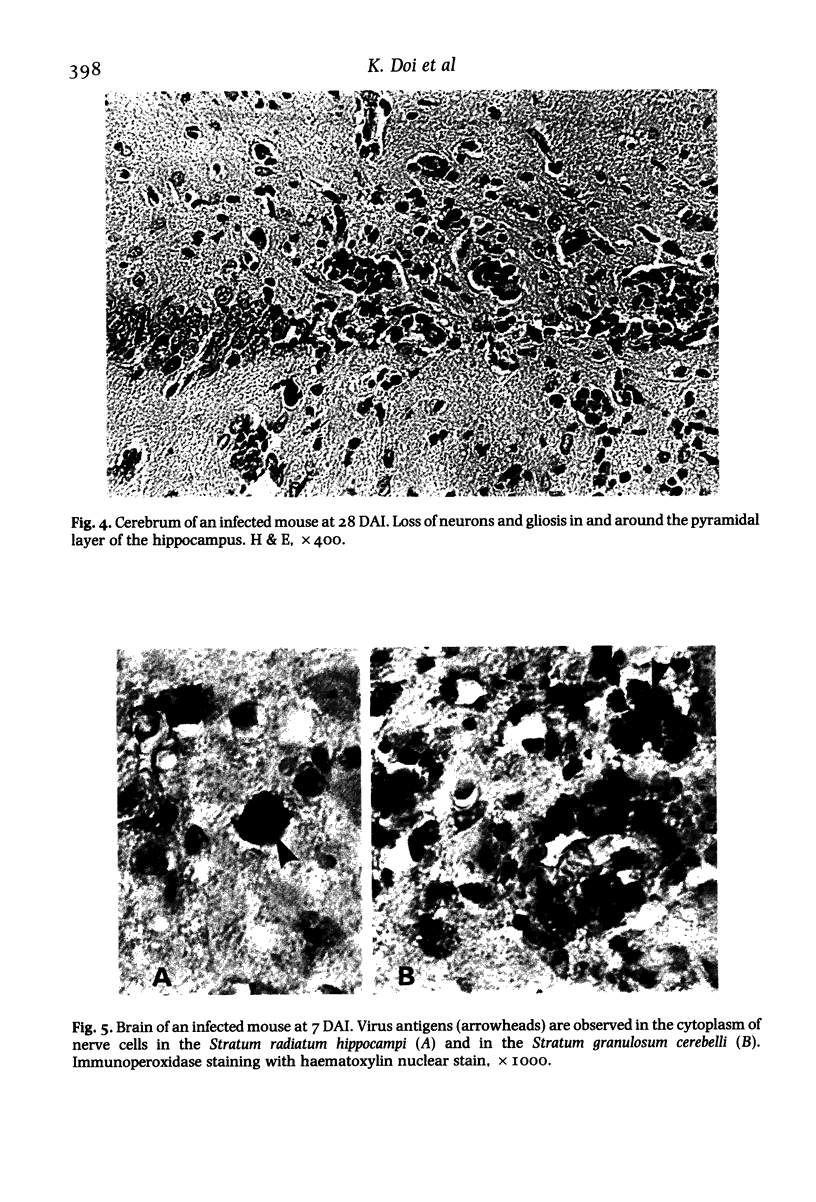
BALB/c mice infected with 10(5) pfu of the D variant of encephalomyocarditis virus were examined histopathologically during the subacute stage of infection. Main pathologic changes were observed in the brain, heart (massive myocardial necrosis with subsequent organization (i.e., replacement of necrotic myocardium by connective tissue) and congestion and dilatation of the right ventricle) and pancreas (moderate degranulation of beta-cells resulting in elevation of blood glucose level). The brain lesions were found most frequently in the pyramidal layer of the hippocampus and the granular layer of the cerebellum and were characterized by degeneration of neurons containing virus antigens. Perivascular mononuclear cell infiltration, spreading to the adjacent brain tissue, and thrombosis in small vessels were also frequently seen. Focal loss of neurons and gliosis developed later in these lesions.
Full text
PDF

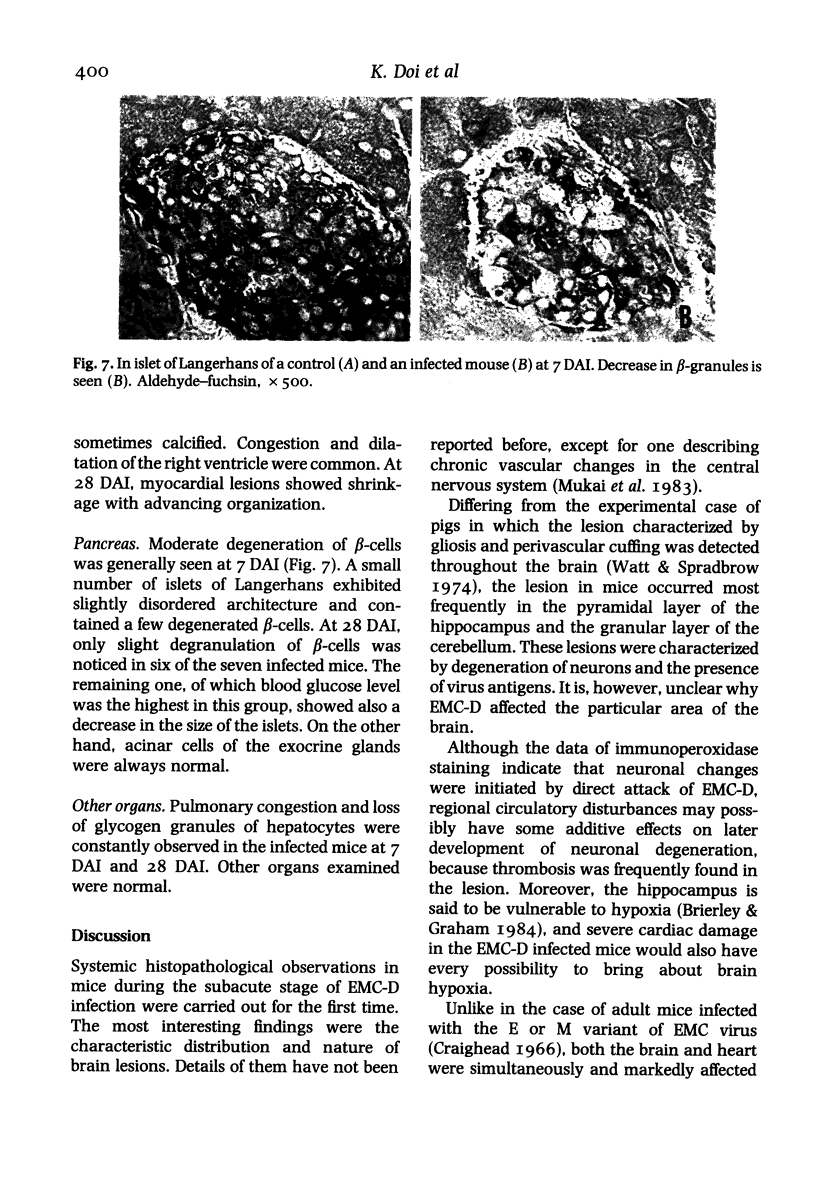

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burch G. E., Harb J. M. Characteristic ultrastructural changes in organs of newborn mice infected with EMC virus. Proc Soc Exp Biol Med. 1974 Sep;146(4):1076–1086. doi: 10.3181/00379727-146-38249. [DOI] [PubMed] [Google Scholar]
- Craighead J. E., McLane M. F. Diabetes mellitus: induction in mice by encephalomyocarditis virus. Science. 1968 Nov 22;162(3856):913–914. doi: 10.1126/science.162.3856.913. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- Helwig F. C., Schmidt C. H. A FILTER-PASSING AGENT PRODUCING INTERSTITIAL MYOCARDITIS IN ANTHROPOID APES AND SMALL ANIMALS. Science. 1945 Jul 13;102(2637):31–33. doi: 10.1126/science.102.2637.31. [DOI] [PubMed] [Google Scholar]
- MURNANE T. G., CRAIGHEAD J. E., MONDRAGON H., SHELOKOV A. Fatal disease of swine due to encephalomyocarditis virus. Science. 1960 Feb 19;131(3399):498–499. doi: 10.1126/science.131.3399.498. [DOI] [PubMed] [Google Scholar]
- Matsumori A., Kawai C. An animal model of congestive (dilated) cardiomyopathy: dilatation and hypertrophy of the heart in the chronic stage in DBA/2 mice with myocarditis caused by encephalomyocarditis virus. Circulation. 1982 Aug;66(2):355–360. doi: 10.1161/01.cir.66.2.355. [DOI] [PubMed] [Google Scholar]
- Yoon J. W., McClintock P. R., Onodera T., Notkins A. L. Virus-induced diabetes mellitus. XVIII. Inhibition by a nondiabetogenic variant of encephalomyocarditis virus. J Exp Med. 1980 Oct 1;152(4):878–892. doi: 10.1084/jem.152.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. W., Rodrigues M. M., Currier C., Notkins A. L. Long-term complications of virus-induced diabetes mellitus in mice. Nature. 1982 Apr 8;296(5857):566–569. doi: 10.1038/296566a0. [DOI] [PubMed] [Google Scholar]