Abstract
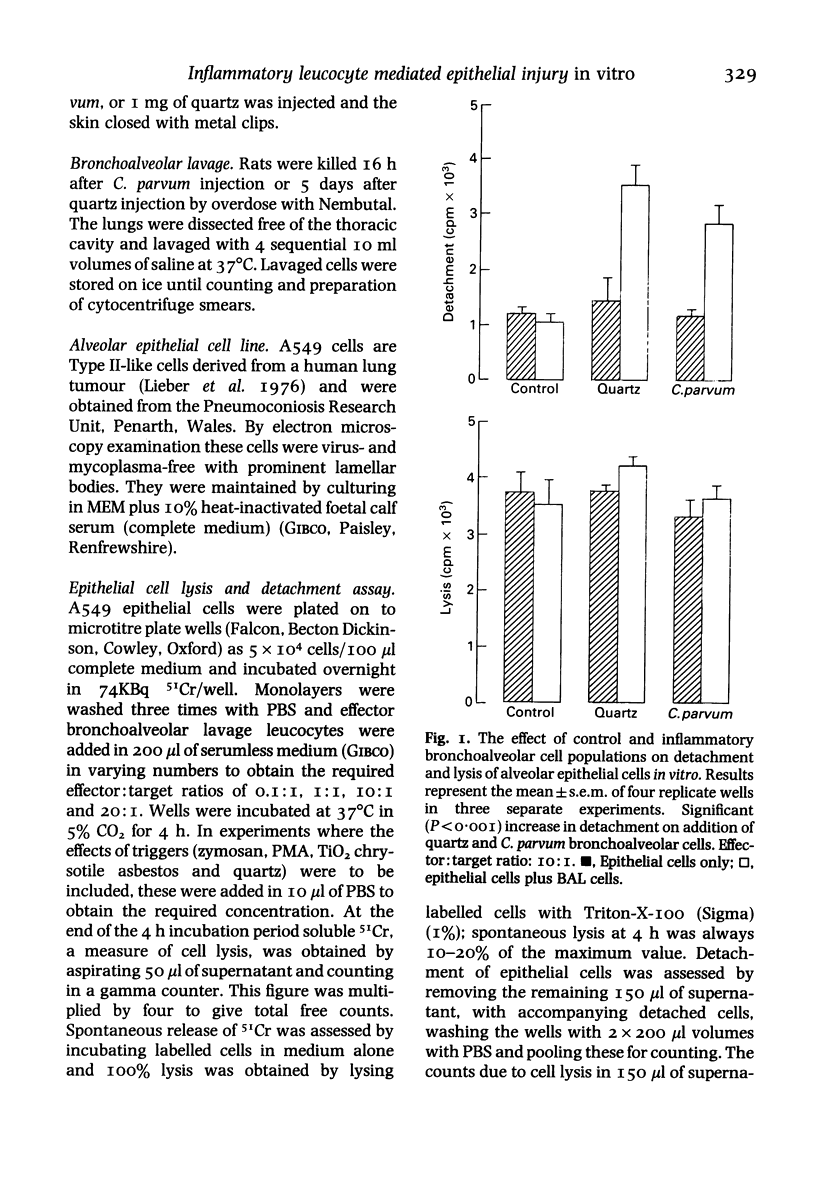
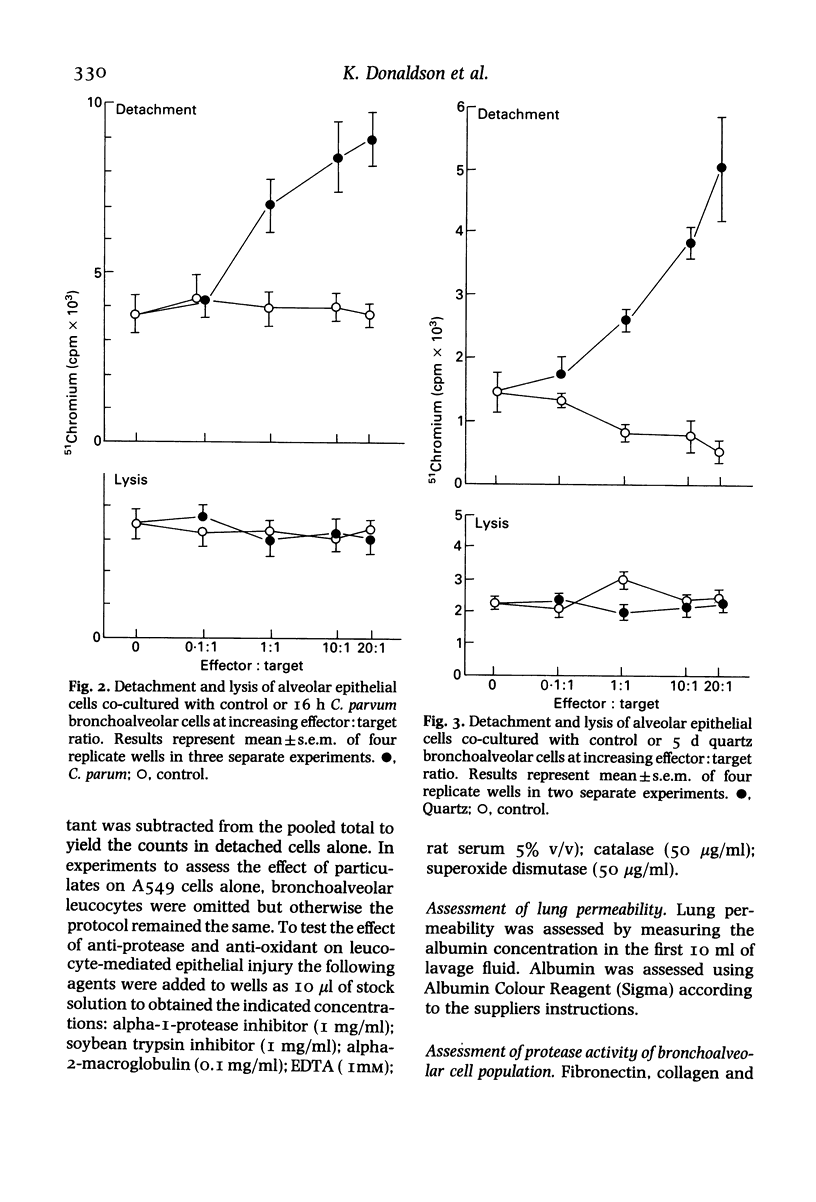
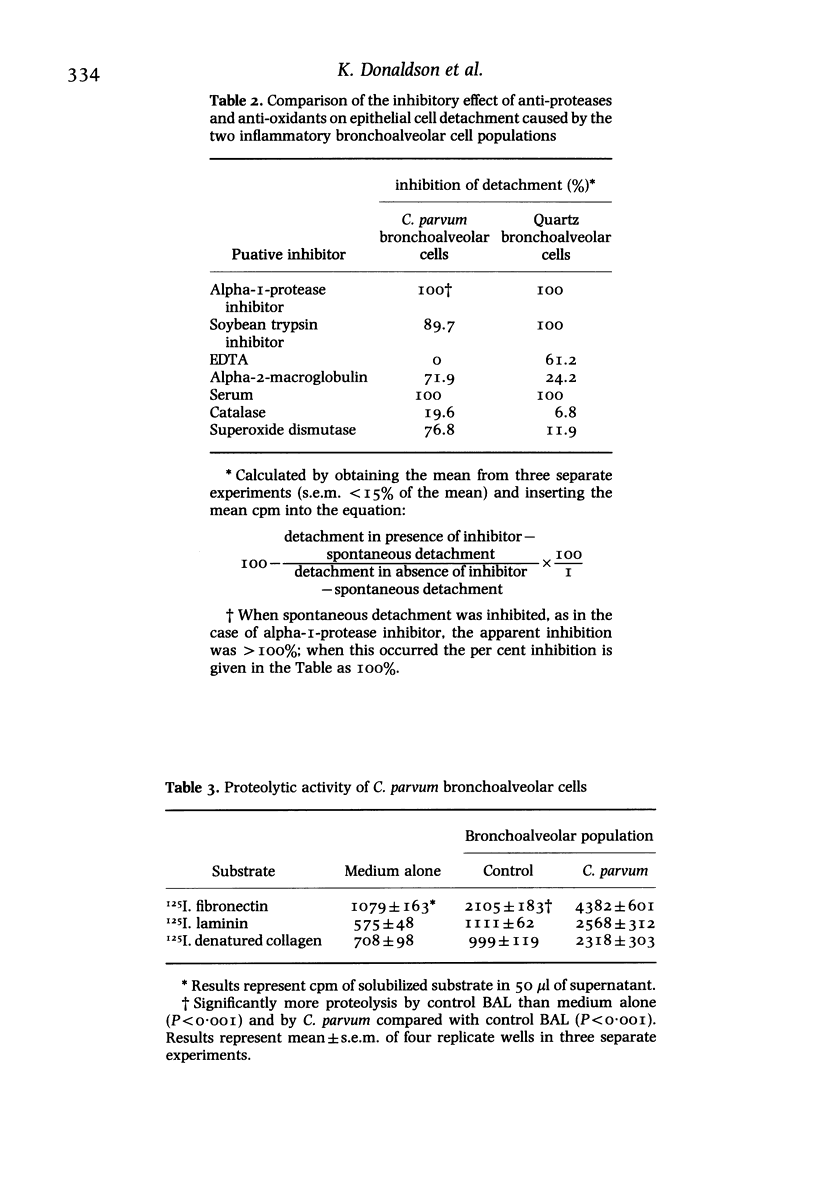
Inflammatory cells are recruited to the parenchyma of the lung in a range of conditions where they are considered to have the ability to exert damaging effects on elements of the alveolus. The injurious effects of rat bronchoalveolar-derived inflammatory cells on an alveolar Type II epithelial cell line were therefore assessed. Inflammatory populations produced by intratracheal injection of Corynebacterium parvum or quartz caused non-lethal detachment injury to the epithelial cells on co-culture whereas control bronchoalveolar cells had no effect on epithelial cells. The pathogenic mineral dusts quartz and chrysotile asbestos caused increased detachment injury when added to co-cultures of epithelial cells and bronchoalveolar leucocyte populations; neither titanium dioxide, a control mineral dust, nor zymosan were active in this respect. Detachment injury was particularly marked when quartz was added to co-cultures of epithelial cells and inflammatory bronchoalveolar cells from quartz treated lung. On the basis of anti-protease and anti-oxidant studies, the detachment injury was found to be mediated by protease alone in the case of quartz cells and protease plus oxidant in the case of C. parvum cells. The two inflammatory bronchoalveolar cell populations were found to have increased proteolytic activity, compared to control bronchoalveolar cells, as shown by increased ability to degrade fibronectin, laminin and denatured collagen. Inflammatory bronchoalveolar cells therefore have the potential to attack elements of the septal extracellular matrix as well as to compromise the integrity of the alveolar epithelium.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Dewald B. The neutrophil. Int Arch Allergy Appl Immunol. 1985;76 (Suppl 1):13–20. doi: 10.1159/000233730. [DOI] [PubMed] [Google Scholar]
- Bégin R., Rola-Pleszczynski M., Massé S., Lemaire I., Sirois P., Boctor M., Nadeau D., Drapeau G., Bureau M. A. Asbestos-induced lung injury in the sheep model: the initial alveolitis. Environ Res. 1983 Feb;30(1):195–210. doi: 10.1016/0013-9351(83)90180-9. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Gadek J. E., Ferrans V. J., Fulmer J. D., Line B. R., Hunninghake G. W. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981 Mar;70(3):542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- Doll N. J., Stankus R. P., Barkman H. W. Immunopathogenesis of asbestosis, silicosis, and coal workers' pneumoconiosis. Clin Chest Med. 1983 Jan;4(1):3–14. [PubMed] [Google Scholar]
- Gellert A. R., Langford J. A., Winter R. J., Uthayakumar S., Sinha G., Rudd R. M. Asbestosis: assessment by bronchoalveolar lavage and measurement of pulmonary epithelial permeability. Thorax. 1985 Jul;40(7):508–514. doi: 10.1136/thx.40.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppleston A. G. Animal model of human disease. Pulmonary alveolar lipo-proteinosis. Animal model: Silica-induced pulmonary alveolar lipo-proteinosis. Am J Pathol. 1975 Jan;78(1):171–174. [PMC free article] [PubMed] [Google Scholar]
- Hoidal J. R., Niewoehner D. E. Pathogenesis of emphysema. Chest. 1983 Apr;83(4):679–685. doi: 10.1378/chest.83.4.679. [DOI] [PubMed] [Google Scholar]
- Lieber M., Smith B., Szakal A., Nelson-Rees W., Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976 Jan 15;17(1):62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- Lugano E. M., Dauber J. H., Daniele R. P. Acute experimental silicosis. Lung morphology, histology, and macrophage chemotaxin secretion. Am J Pathol. 1982 Oct;109(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., 2nd Neutrophils kill pulmonary endothelial cells by a hydrogen-peroxide-dependent pathway. An in vitro model of neutrophil-mediated lung injury. Am Rev Respir Dis. 1984 Aug;130(2):209–213. doi: 10.1164/arrd.1984.130.2.209. [DOI] [PubMed] [Google Scholar]
- Riley D. J., Kerr J. S. Oxidant injury of the extracellular matrix: potential role in the pathogenesis of pulmonary emphysema. Lung. 1985;163(1):1–13. doi: 10.1007/BF02713801. [DOI] [PubMed] [Google Scholar]
- Rinderknecht J., Shapiro L., Krauthammer M., Taplin G., Wasserman K., Uszler J. M., Effros R. M. Accelerated clearance of small solutes from the lungs in interstitial lung disease. Am Rev Respir Dis. 1980 Jan;121(1):105–117. doi: 10.1164/arrd.1980.121.1.105. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler M. R., Gaumer H. R., Stankus R. P., Kaimal J., Hoffmann E., Salvaggio J. E. Bronchoalveolar lavage in silicosis. Evidence of type II cell hyperplasia. Lung. 1980;157(2):95–102. [PubMed] [Google Scholar]
- Sugahara K., Cott G. R., Parsons P. E., Mason R. J., Sandhaus R. A., Henson P. M. Epithelial permeability produced by phagocytosing neutrophils in vitro. Am Rev Respir Dis. 1986 May;133(5):875–881. [PubMed] [Google Scholar]
- Taubman S. B., Cogen R. B. Cell-detaching activity mediated by an enzyme(s) obtained from human leukocyte granules. Lab Invest. 1975 Apr;32(4):555–560. [PubMed] [Google Scholar]
- Weiland J. E., Davis W. B., Holter J. F., Mohammed J. R., Dorinsky P. M., Gadek J. E. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986 Feb;133(2):218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]


