Abstract
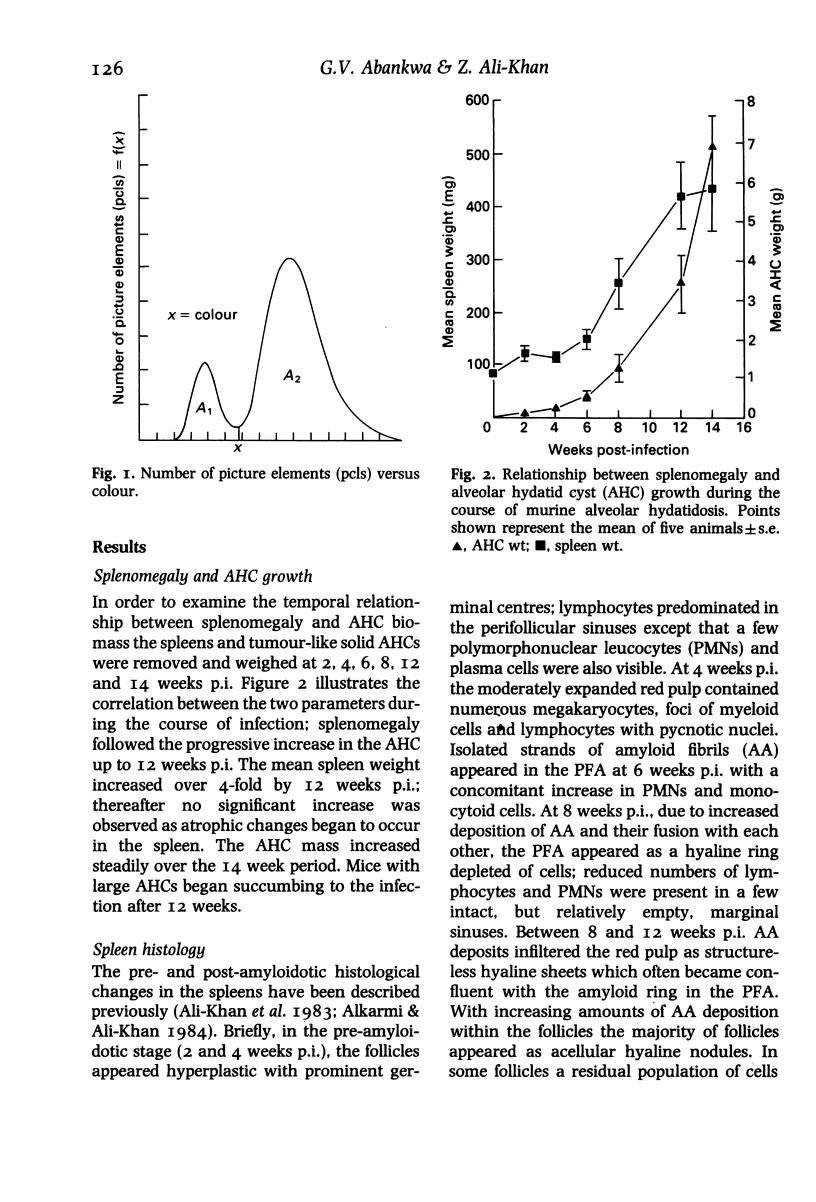
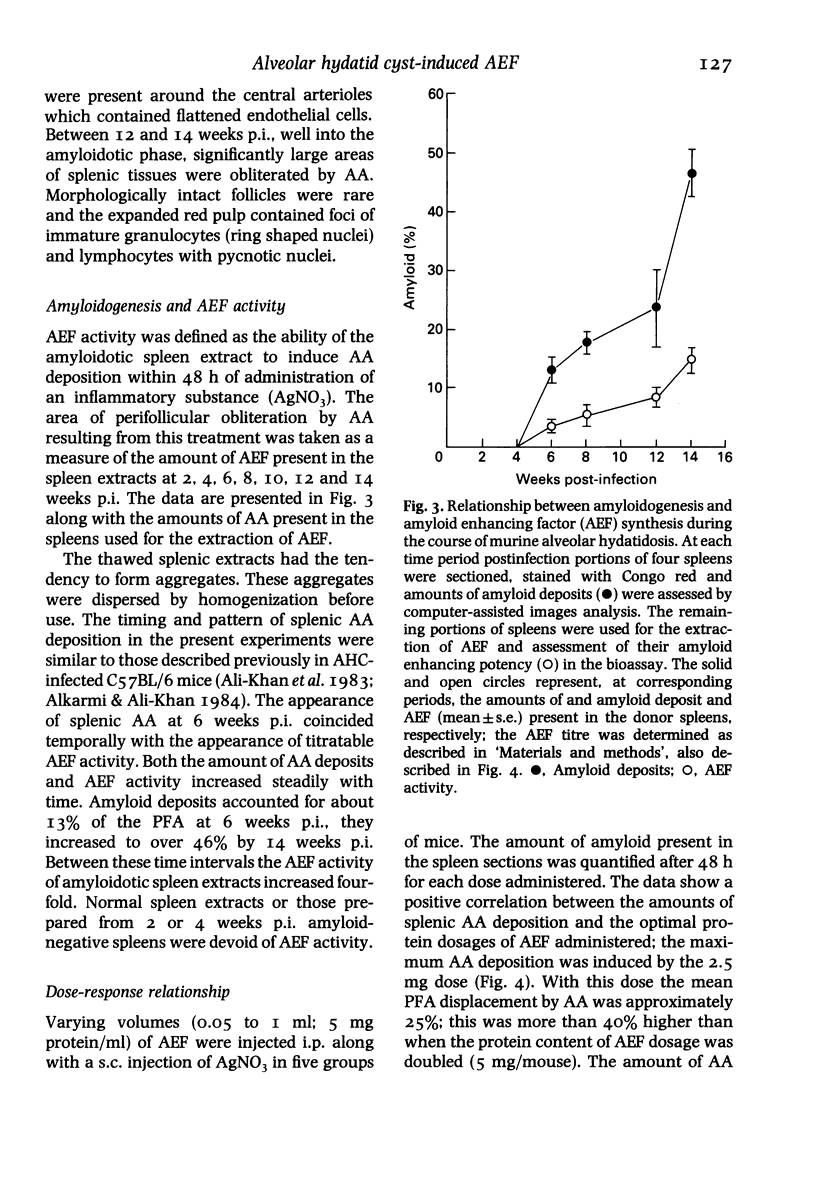
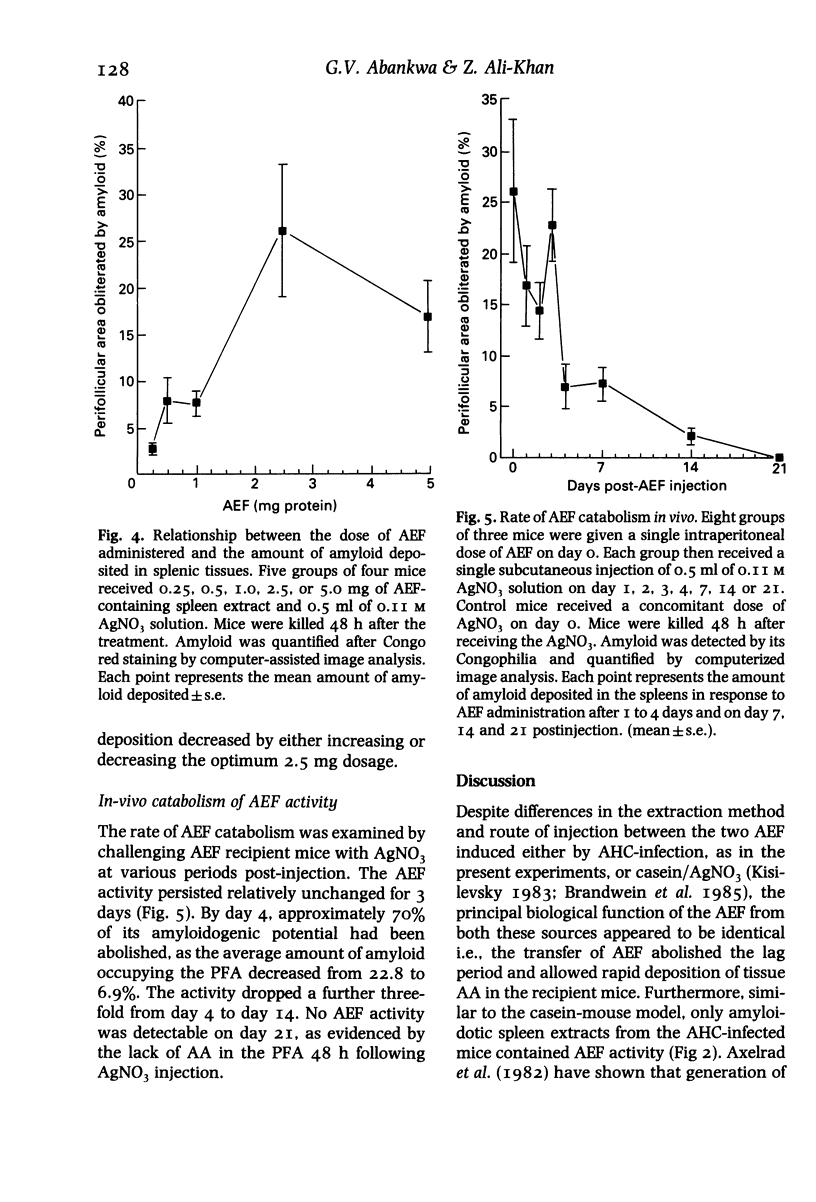
The biological activity and time of appearance of alveolar hydatid cyst induced splenic amyloid enhancing factor (AEF) with respect to amyloid deposition in the spleens were determined in C57BL/6J mice. Mice were infected intraperitoneally with 100 alveolar hydatid cysts (AHC) and killed bi-weekly between 2 and 14 weeks postinfection (p.i.). AHCs and spleens were excised, weighed and a portion of each spleen was sectioned and stained for quantitation of amyloid deposits and histological studies. The remaining spleen pieces were sonicated separately in cold phosphate buffered saline, pH 7.4 (I g/10 ml), centrifuged (27,000 g, 60 min, 4 degrees C) and the supernatant tested for AEF activity. Splenomegaly followed the progressive increase in the AHC biomass and AEF activity coincided with the appearance of amyloid deposits at 6 weeks p.i. A 2.5 mg intraperitoneal protein dosage of AEF in conjunction with a subcutaneous injection of 0.5 ml of a 0.11 M AgNO3 solution in mice, induced the maximum amount of splenic amyloid deposition at 48 h; the amount of splenic amyloid deposits decreased by either increasing or decreasing the AEF dosage. In vivo, 70% of the AEF activity was abolished by day 4 post-injection of AEF and completely by 3 weeks. These findings indicate that AHC-induced AEF is functionally analogous to casein-induced AEF and its appearance in the spleen coincides with neutrophilia, histiocytosis and amyloid deposition.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abankwa G. V., Ali-Khan Z. Alveolar hydatid cyst induced amyloid enhancing factor (AEF): physicochemical properties and abolition of AEF activity by serine protease inhibitors. Br J Exp Pathol. 1988 Feb;69(1):133–148. [PMC free article] [PubMed] [Google Scholar]
- Ali-Khan Z. Cellular changes in the lymphoreticular tissues of C57L/J mice infected with Echinococcus multilocularis cysts. Immunology. 1978 May;34(5):831–839. [PMC free article] [PubMed] [Google Scholar]
- Ali-Khan Z. Host-parasite relationship in echinococcosis. II. Cyst weight, hematologic alterations, and gross changes in the spleen and lymph nodes of C57L mice against graded doses of Echinococcus multilocularis cysts. J Parasitol. 1974 Apr;60(2):236–242. [PubMed] [Google Scholar]
- Ali-Khan Z., Jothy S., Al-Karmi T. Murine alveolar hydatidosis: a potential experimental model for the study of AA-amyloidosis. Br J Exp Pathol. 1983 Dec;64(6):599–611. [PMC free article] [PubMed] [Google Scholar]
- Ali-Khan Z., Rausch R. L. Demonstration of amyloid and immune complex deposits in renal and hepatic parenchyma of Alaskan alveolar hydatid disease patients. Ann Trop Med Parasitol. 1987 Aug;81(4):381–392. doi: 10.1080/00034983.1987.11812136. [DOI] [PubMed] [Google Scholar]
- Ali-Khan Z., Siboo R. Echinococcus multilocularis: immunoglobulin and antibody response in C57BL/6J mice. Exp Parasitol. 1982 Feb;53(1):97–104. doi: 10.1016/0014-4894(82)90096-0. [DOI] [PubMed] [Google Scholar]
- Alkarmi T. O., Ali-Khan Z. Chronic alveolar hydatidosis and secondary amyloidosis: pathological aspects of the disease in four strains of mice. Br J Exp Pathol. 1984 Aug;65(4):405–417. [PMC free article] [PubMed] [Google Scholar]
- Alkarmi T. O., Ali-Khan Z., Zarkadas C. G. Characterization of amyloid protein from mice infected with alveolar hydatid cyst: isolation, purification, and amino acid composition. Exp Mol Pathol. 1986 Oct;45(2):142–159. doi: 10.1016/0014-4800(86)90055-9. [DOI] [PubMed] [Google Scholar]
- Axelrad M. A., Kisilevsky R., Willmer J., Chen S. J., Skinner M. Further characterization of amyloid-enhancing factor. Lab Invest. 1982 Aug;47(2):139–146. [PubMed] [Google Scholar]
- Brandwein S. R., Sipe J. D., Skinner M., Cohen A. S. Effect of colchicine on experimental amyloidosis in two CBA/J mouse models. Chronic inflammatory stimulation and administration of amyloid-enhancing factor during acute inflammation. Lab Invest. 1985 Mar;52(3):319–325. [PubMed] [Google Scholar]
- Fritz H., Jochum M., Geiger R., Duswald K. H., Dittmer H., Kortmann H., Neumann S., Lang H. Granulocyte proteinases as mediators of unspecific proteolysis in inflammation: a review. Folia Histochem Cytobiol. 1986;24(2):99–115. [PubMed] [Google Scholar]
- Fuks A., Zucker-Franklin D. Impaired Kupffer cell function precedes development of secondary amyloidosis. J Exp Med. 1985 May 1;161(5):1013–1028. doi: 10.1084/jem.161.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazimierczak J. Cytochemical study of casein-induced and nitrogen mustard accelerated amyloidosis in mice. Acta Pathol Microbiol Scand. 1969;77(2):201–217. doi: 10.1111/j.1699-0463.1969.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Kisilevsky R. Amyloidosis: a familiar problem in the light of current pathogenetic developments. Lab Invest. 1983 Oct;49(4):381–390. [PubMed] [Google Scholar]
- Lavie G., Zucker-Franklin D., Franklin E. C. Degradation of serum amyloid A protein by surface-associated enzymes of human blood monocytes. J Exp Med. 1978 Oct 1;148(4):1020–1031. doi: 10.1084/jem.148.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Secretion of lysosomal hydrolases by stimulated and nonstimulated macrophages. J Exp Med. 1978 Aug 1;148(2):435–450. doi: 10.1084/jem.148.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman S. L., Cathcart E. S., Skinner M., Cohen A. S. The degradation of serum amyloid A protein by activated polymorphonuclear leucocytes: participation of granulocytic elastase. Immunology. 1982 Aug;46(4):737–744. [PMC free article] [PubMed] [Google Scholar]
- Taichman N. S., Pruzanski W., Ranadive N. S. Release of intracellular constituents from rabbit polymorphonuclear leukocytes exposed to soluble and insoluble immune complexes. Int Arch Allergy Appl Immunol. 1972;43(2):182–195. doi: 10.1159/000230836. [DOI] [PubMed] [Google Scholar]
- Treves S., Ali-Khan Z. Characterization of the inflammatory cells in progressing tumor-like alveolar hydatid cyst. 2. Cell surface receptors, endocytosed immune complexes and lysosomal enzyme content. Tropenmed Parasitol. 1984 Dec;35(4):231–236. [PubMed] [Google Scholar]
- Treves S., Ali-Khan Z. Characterization of the inflammatory cells in progressing tumor-like alveolar hydatid cysts: 1. Kinetics and composition of inflammatory infiltrates. Tropenmed Parasitol. 1984 Sep;35(3):183–188. [PubMed] [Google Scholar]
- Turner R. A., Schumacher R., Myers A. R. Phagocytic function of polymorphonuclear leukocytes in rheumatic diseases. J Clin Invest. 1973 Jul;52(7):1632–1635. doi: 10.1172/JCI107342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuitton D. A., Lasségue A., Miguet J. P., Hervé P., Barale T., Seillés E., Capron A. Humoral and cellular immunity in patients with hepatic alveolar echinococcosis. A 2 year follow-up with and without flubendazole treatment. Parasite Immunol. 1984 Jul;6(4):329–340. doi: 10.1111/j.1365-3024.1984.tb00805.x. [DOI] [PubMed] [Google Scholar]


