Abstract
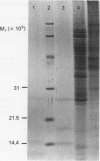
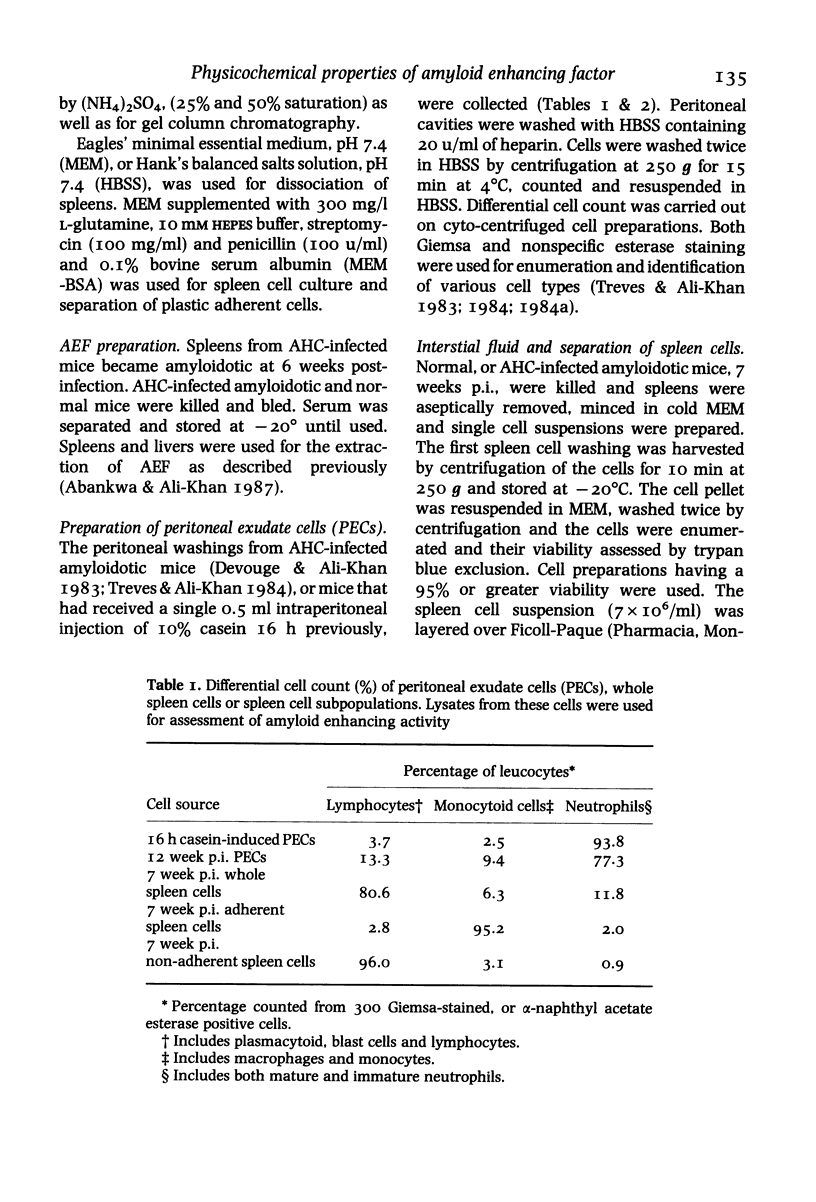
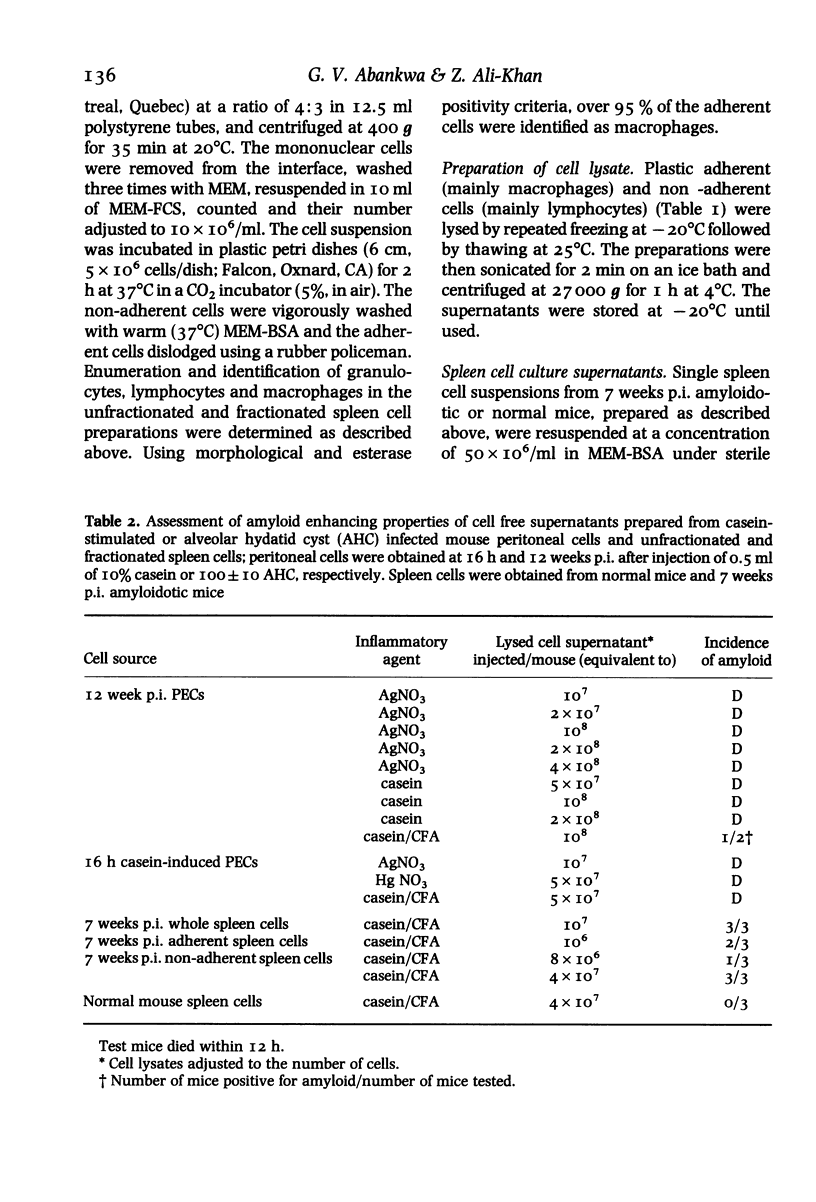
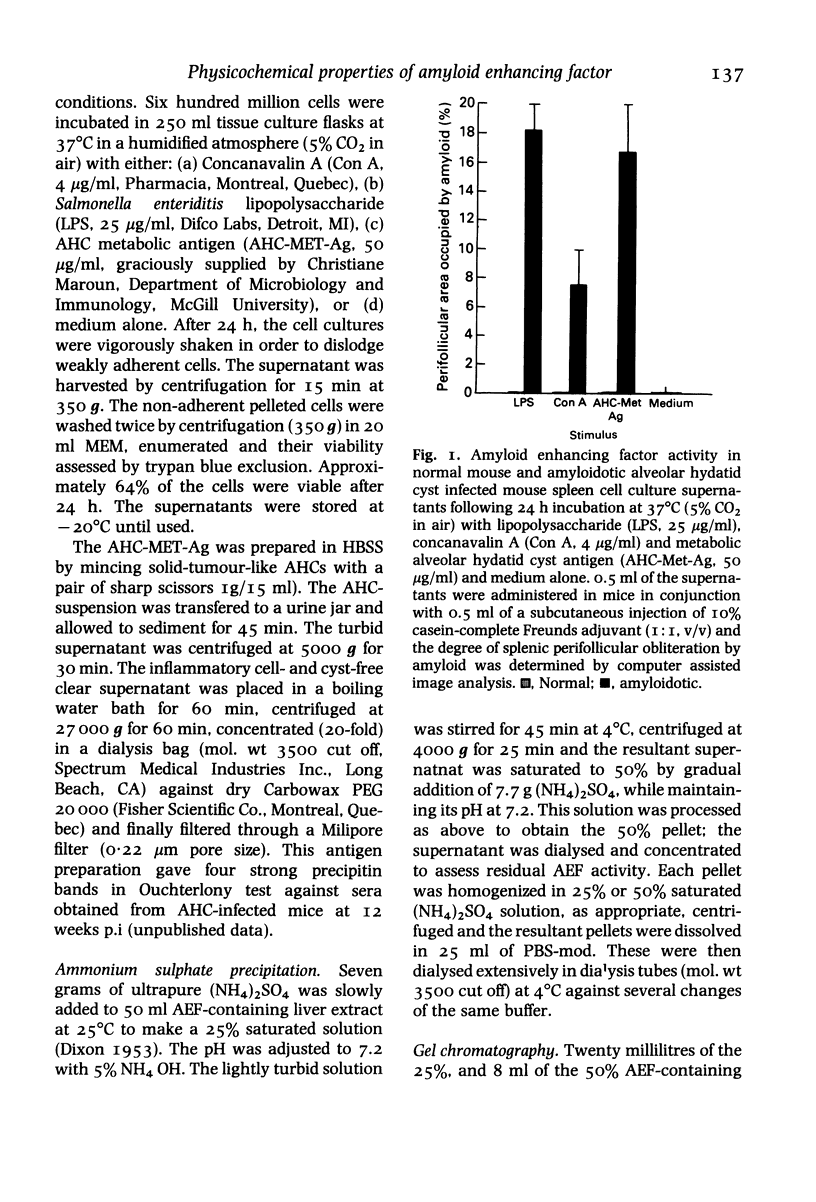
Cell free supernatants prepared from amyloidotic liver, unfractionated and fractionated peritoneal and spleen cells from casein stimulated (16 h post-injection) or alveolar hydatid cyst-infected (7 or 12 weeks post-infection, p. i.) C57BL/6J mice were used to assess amyloid enhancing factor (AEF) activity and to determine its cell-source and physicochemical properties. Of the various supernatants used, the plastic adherent spleen cell lysate (95% macrophages) from 7 weeks p.i mice showed greater AEF activity, on a cell to cell basis, than the lysates prepared from whole spleen cells, peritoneal exudate cells or nonadherent (96% lymphocytes) spleen cells. Culture supernatants obtained from Con A, LPS or the parasite antigen stimulated amyloidotic spleen cells but not the normal mouse spleen cells contained AEF activity; the supernatant from unstimulated amyloidotic spleen cells was negative for AEF activity. AEF was precipitated with 25% and 50% saturation with (NH4)2SO4 and after gel-filtration the low molecular weight fraction contained the AEF activity which on SDS-PAGE resolved into three peptides ranging between mol. wts 15,000 and 31,000. Of the various specific and nonspecific protease inhibitors tested, AEF activity was completely abolished by 30 min preincubation with 10 mM phenylmethylsulphonyl fluoride. Taken together, these results indicate that AEF may be a small molecular weight lysosomal neutral protease of neutrophil/macrophage origin.
Full text
PDF

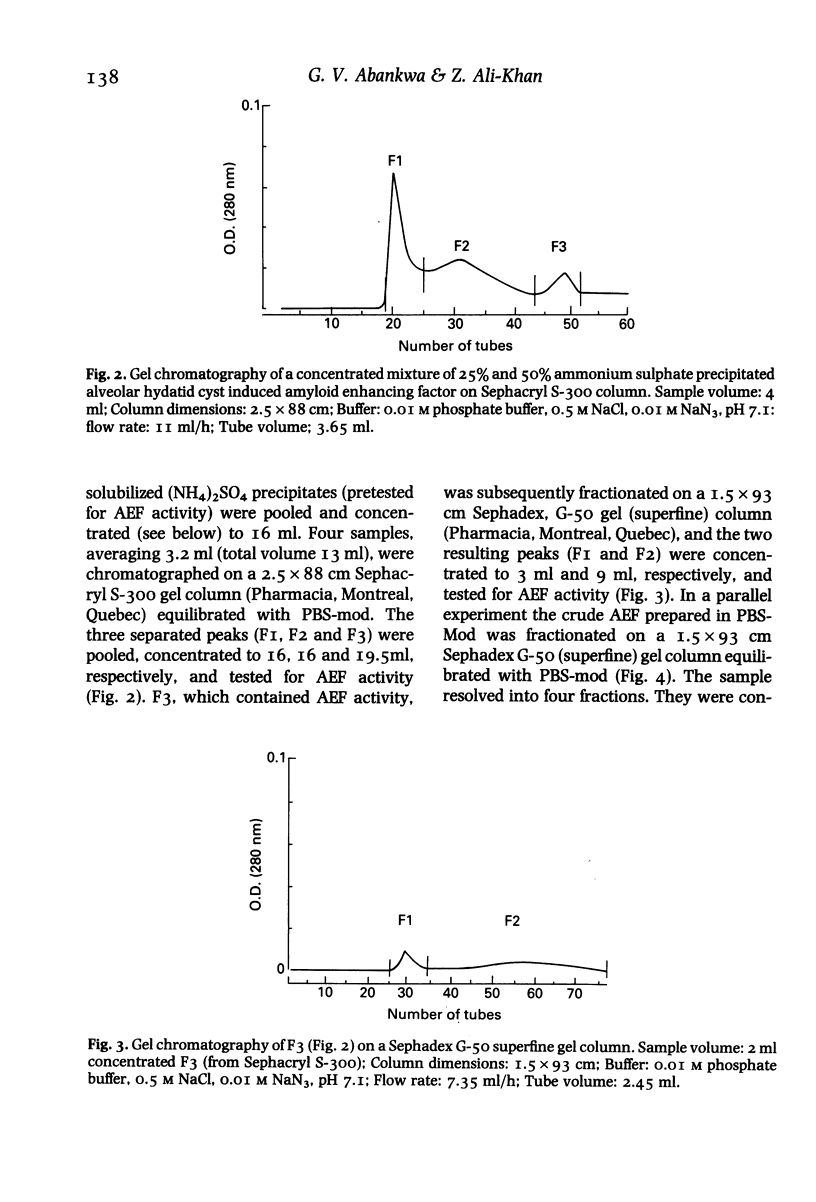
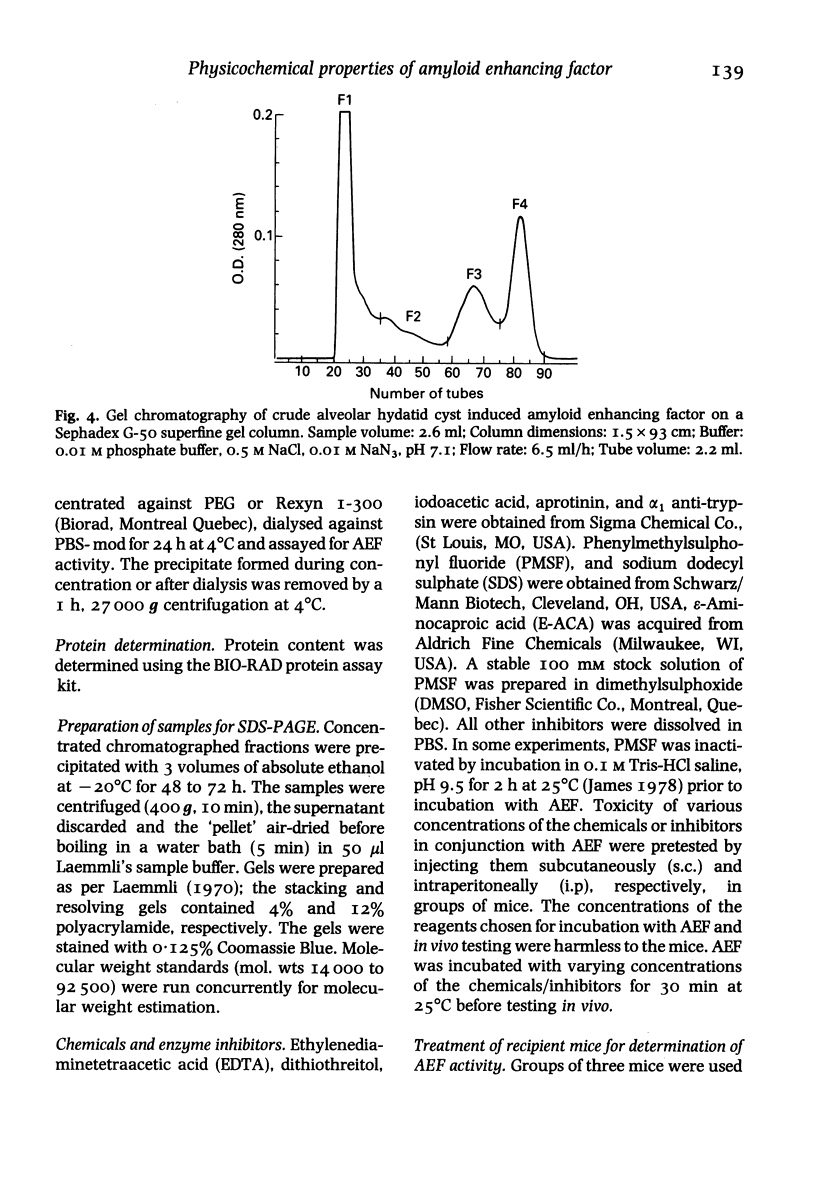
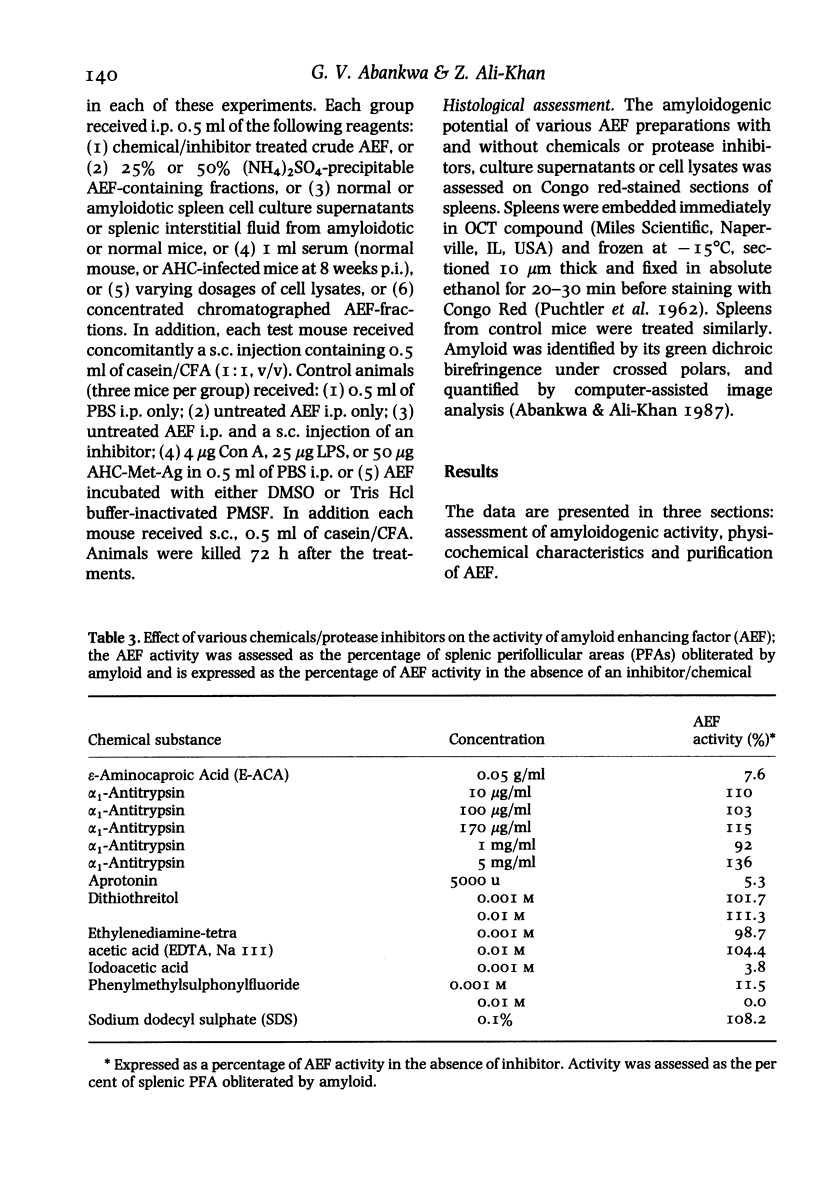
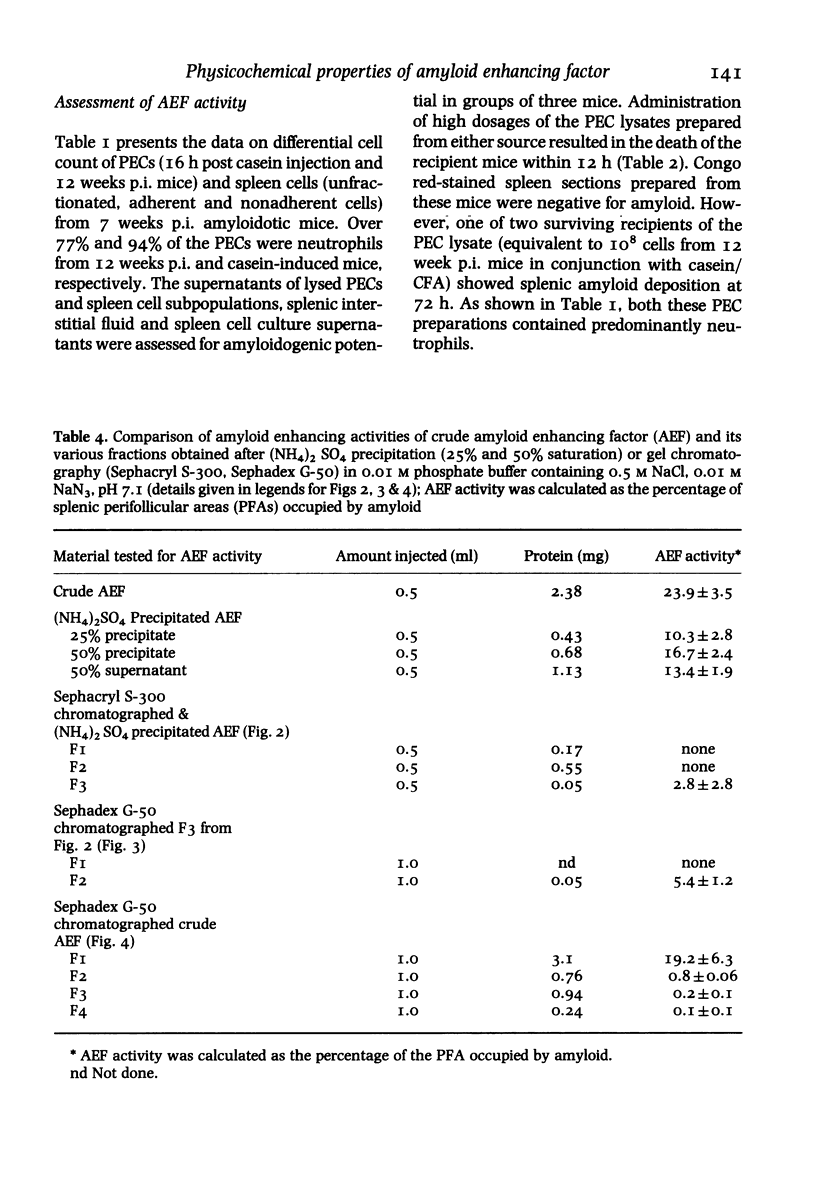






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abankwa G. V., Ali-Khan Z. Induction of amyloid enhancing factor and its biological properties in murine alveolar hydatidosis. Br J Exp Pathol. 1988 Feb;69(1):123–132. [PMC free article] [PubMed] [Google Scholar]
- Ali-Khan Z. Cellular changes in the lymphoreticular tissues of C57L/J mice infected with Echinococcus multilocularis cysts. Immunology. 1978 May;34(5):831–839. [PMC free article] [PubMed] [Google Scholar]
- Ali-Khan Z., Jothy S., Al-Karmi T. Murine alveolar hydatidosis: a potential experimental model for the study of AA-amyloidosis. Br J Exp Pathol. 1983 Dec;64(6):599–611. [PMC free article] [PubMed] [Google Scholar]
- Alkarmi T. O., Ali-Khan Z., Zarkadas C. G. Characterization of amyloid protein from mice infected with alveolar hydatid cyst: isolation, purification, and amino acid composition. Exp Mol Pathol. 1986 Oct;45(2):142–159. doi: 10.1016/0014-4800(86)90055-9. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A., Rocha H. Schistosomal glomerulopathy. Kidney Int. 1979 Jul;16(1):23–29. doi: 10.1038/ki.1979.99. [DOI] [PubMed] [Google Scholar]
- Axelrad M. A., Kisilevsky R., Willmer J., Chen S. J., Skinner M. Further characterization of amyloid-enhancing factor. Lab Invest. 1982 Aug;47(2):139–146. [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. J., Karnovsky M. L. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Jan;48(1):187–192. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart E. S., Rodgers O. G., Cohen A. S. Amyloid-inducing factor and immunological unresponsiveness. Ann Rheum Dis. 1972 Jul;31(4):303–307. doi: 10.1136/ard.31.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. S., Shirahama T., Sipe J. D., Skinner M. Amyloid proteins, precursors, mediator, and enhancer. Lab Invest. 1983 Jan;48(1):1–4. [PubMed] [Google Scholar]
- DIXON M. A nomogram for ammonium sulphate solutions. Biochem J. 1953 Jun;54(3):457–458. doi: 10.1042/bj0540457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H., Jochum M., Geiger R., Duswald K. H., Dittmer H., Kortmann H., Neumann S., Lang H. Granulocyte proteinases as mediators of unspecific proteolysis in inflammation: a review. Folia Histochem Cytobiol. 1986;24(2):99–115. [PubMed] [Google Scholar]
- GOLD A. M. SULFONYL FLUORIDES AS INHIBITORS OF ESTERASES. 3. IDENTIFICATION OF SERINE AS THE SITE OF SULFONYLATION IN PHENYLMETHANESULFONYL ALPHA-CHYMOTRYPSIN. Biochemistry. 1965 May;4:897–901. doi: 10.1021/bi00881a016. [DOI] [PubMed] [Google Scholar]
- González J. L., Insa F., Novoa C., Pizarro M. Intestinal amyloidosis in hamsters with visceral leishmaniasis. Br J Exp Pathol. 1986 Jun;67(3):353–360. [PMC free article] [PubMed] [Google Scholar]
- Herbert V. Megaloblastic anemias. Lab Invest. 1985 Jan;52(1):3–19. [PubMed] [Google Scholar]
- James G. T. Inactivation of the protease inhibitor phenylmethylsulfonyl fluoride in buffers. Anal Biochem. 1978 Jun 1;86(2):574–579. doi: 10.1016/0003-2697(78)90784-4. [DOI] [PubMed] [Google Scholar]
- Janigan D. T., Druet R. L. Experimental murine amyloidosis in x-irradiated recipients of spleen homogenates or serum from sensitized donors. Am J Pathol. 1968 Feb;52(2):381–390. [PMC free article] [PubMed] [Google Scholar]
- Kisilevsky R. Amyloidosis: a familiar problem in the light of current pathogenetic developments. Lab Invest. 1983 Oct;49(4):381–390. [PubMed] [Google Scholar]
- Kisilevsky R., Axelrad M., Corbett W., Brunet S., Scott F. The role of inflammatory cells in the pathogenesis of amyloidosis. Lab Invest. 1977 Dec;37(6):544–553. [PubMed] [Google Scholar]
- Königk E., Putfarken B. Inhibition of ornithine decarboxylase of in vitro cultured Plasmodium falciparum by chloroquine. Tropenmed Parasitol. 1983 Mar;34(1):1–3. [PubMed] [Google Scholar]
- Lavie G., Zucker-Franklin D., Franklin E. C. Degradation of serum amyloid A protein by surface-associated enzymes of human blood monocytes. J Exp Med. 1978 Oct 1;148(4):1020–1031. doi: 10.1084/jem.148.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranlov P. The adoptive transfer of experimental mouse amyloidosis by intravenous injections of spleen cell extracts from casein-treated syngeneic donor mice. Acta Pathol Microbiol Scand. 1967;70(3):321–335. [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Secretion of lysosomal hydrolases by stimulated and nonstimulated macrophages. J Exp Med. 1978 Aug 1;148(2):435–450. doi: 10.1084/jem.148.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman S. L., Cathcart E. S., Skinner M., Cohen A. S. The degradation of serum amyloid A protein by activated polymorphonuclear leucocytes: participation of granulocytic elastase. Immunology. 1982 Aug;46(4):737–744. [PMC free article] [PubMed] [Google Scholar]
- Skinner M., Stone P., Shirahama T., Connors L. H., Calore J., Cohen A. S. The association of an elastase with amyloid fibrils. Proc Soc Exp Biol Med. 1986 Feb;181(2):211–214. doi: 10.3181/00379727-181-42242. [DOI] [PubMed] [Google Scholar]
- Skogen B., Natvig J. B. Degradation of amyloid proteins by different serine proteases. Scand J Immunol. 1981 Oct;14(4):389–396. doi: 10.1111/j.1365-3083.1981.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Prostaglandins, macrophages, and immunity. J Immunol. 1980 Jul;125(1):1–5. [PubMed] [Google Scholar]
- Treves S., Ali-Khan Z. Characterization of the inflammatory cells in progressing tumor-like alveolar hydatid cyst. 2. Cell surface receptors, endocytosed immune complexes and lysosomal enzyme content. Tropenmed Parasitol. 1984 Dec;35(4):231–236. [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by endotoxin-activated macrophages. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3598–3601. doi: 10.1073/pnas.71.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Chin J. R. Apoprotein E is synthesized and secreted by resident and thioglycollate-elicited macrophages but not by pyran copolymer- or bacillus Calmette-Guerin-activated macrophages. J Exp Med. 1983 Oct 1;158(4):1272–1293. doi: 10.1084/jem.158.4.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]