Abstract
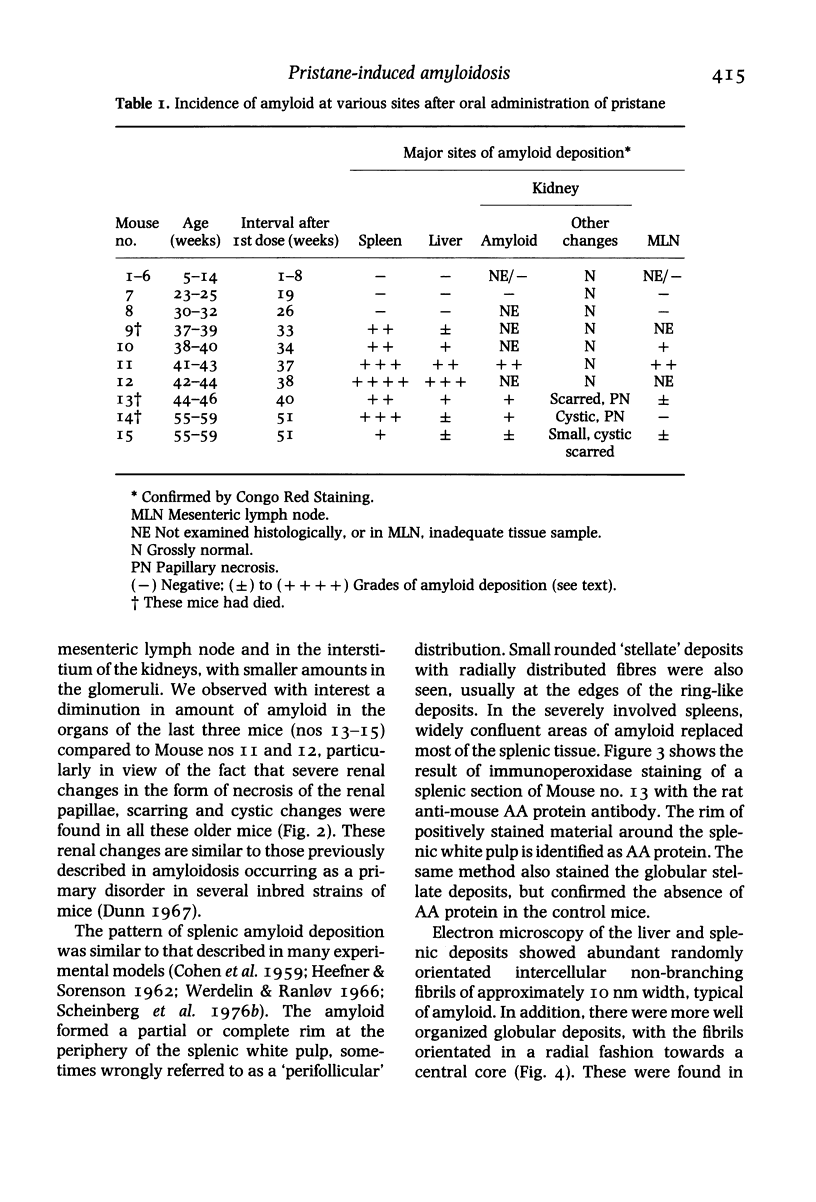
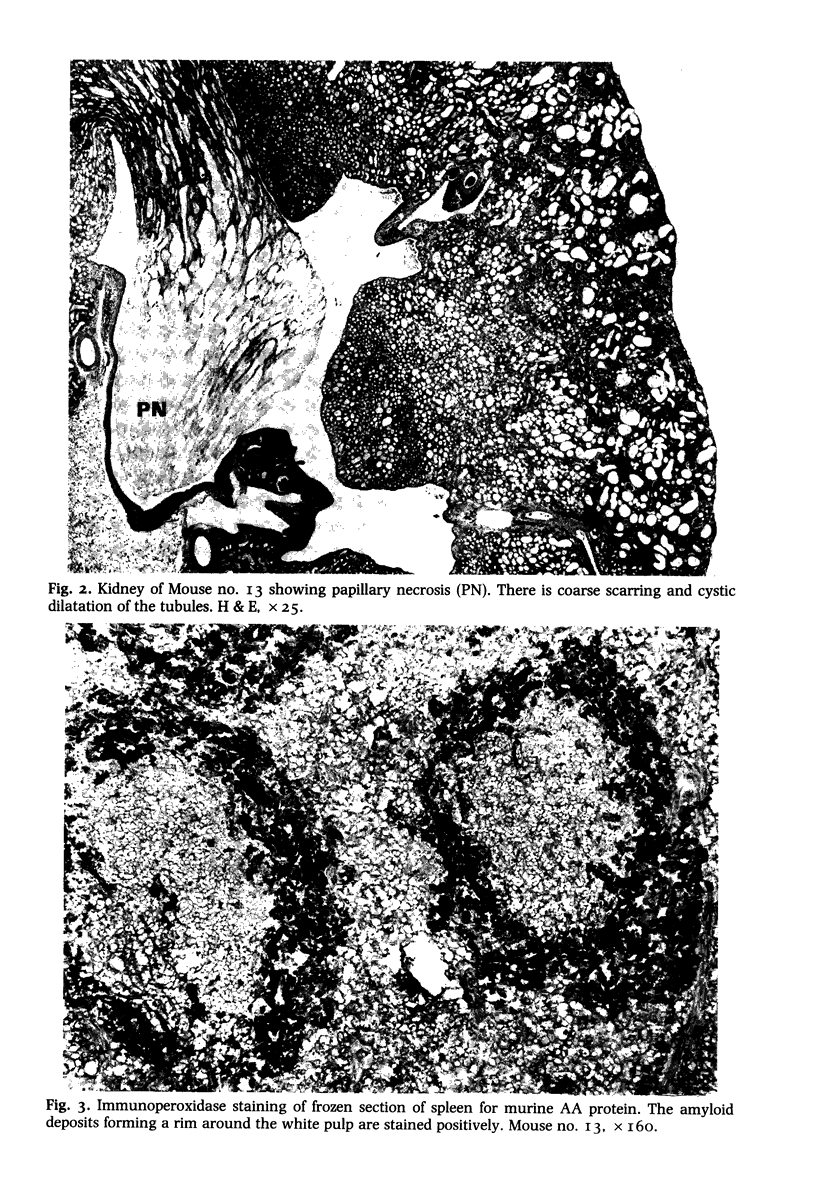
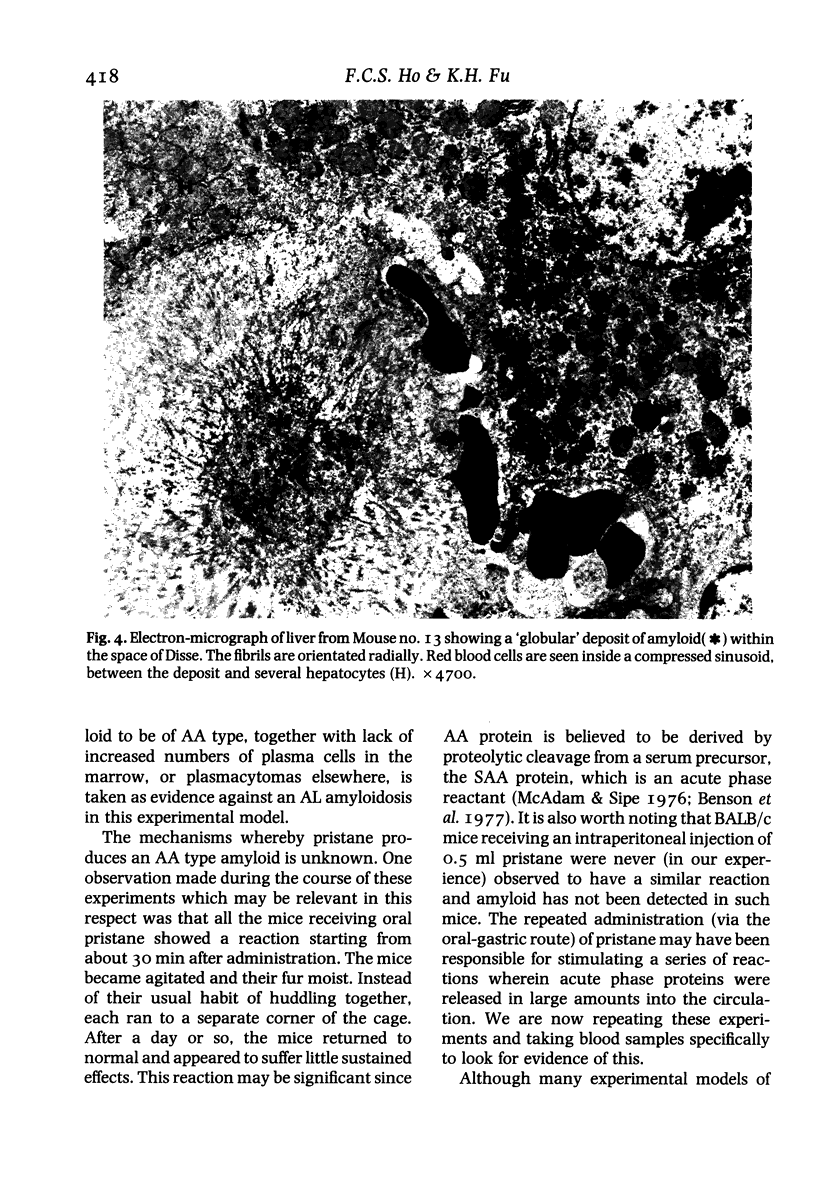
Fifteen male BALB/c mice were given six intermittent oral doses of O.I. ml pristane (2, 6, 10, 14 tetramethylpentadecane) within a period of 9 weeks. Fifteen mice receiving tap water using the same schedule formed the control group. Amyloidosis was first detected in the spleen of a mouse which had died 33 weeks after the first dose and 24 weeks after the last. All six mice which were subsequently autopsied 34-51 weeks after the first dose also showed amyloidosis involving liver and spleen. The most extensive tissue deposits were seen at 37-38 weeks whereas the older mice showed predominantly chronic renal lesions with papillary necrosis, scars and cystic change. Electron microscopy confirmed the identity of the amyloid fibrils and the presence of globular stellate amyloid 'bodies' in liver and spleen. The amyloid deposits were shown to be made up of AA (amyloid associated) protein using an indirect immunoperoxidase method and a monoclonal rat anti-murine AA protein antibody. We did not find any plasmacytomas or increased numbers of plasma cells in the bone marrow. None of the control mice developed amyloidosis. This new experimental model promises to provide a means of studying several aspects of secondary amyloidosis which may be relevant to the clinical situation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avigan J., Milne G. W., Highet R. J. The occurrence of pristane and phytane in man and animals. Biochim Biophys Acta. 1967 Aug 8;144(1):127–131. doi: 10.1016/0005-2760(67)90084-7. [DOI] [PubMed] [Google Scholar]
- Bari W. A., Pettengill O. S., Sorenson G. D. Electron microscopy and electron microscopic autoradiography of splenic cell cultures from mice with amyloidosis. Lab Invest. 1969 Mar;20(3):234–242. [PubMed] [Google Scholar]
- COHEN A. S., CALKINS E., LEVENE C. I. Studies on experimental amyloidosis. I. Analysis of histology and staining reactions of casein-induced amyloidosis in the rabbit. Am J Pathol. 1959 Sep-Oct;35:971–989. [PMC free article] [PubMed] [Google Scholar]
- Cohen A. S., Shirahama T. Animal model for human disease: spontaneous and induced amyloidosis. Am J Pathol. 1972 Aug;68(2):441–444. [PMC free article] [PubMed] [Google Scholar]
- Janigan D. T., Druet R. L. Experimental amyloidosis. Role of antigenicity and rapid induction. Am J Pathol. 1966 Jun;48(6):1013–1025. [PMC free article] [PubMed] [Google Scholar]
- Livni N., Laufer A., Levo Y. Demonstration of amyloid in murine and human secondary amyloidosis by the immunoperoxidase technique. J Pathol. 1980 Dec;132(4):343–348. doi: 10.1002/path.1711320405. [DOI] [PubMed] [Google Scholar]
- McAdam K. P., Sipe J. D. Murine model for human secondary amyloidosis: genetic variability of the acute-phase serum protein SAA response to endotoxins and casein. J Exp Med. 1976 Oct 1;144(4):1121–1127. doi: 10.1084/jem.144.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTER G. W. The resorption of amyloid under experimental conditions. Am J Pathol. 1954 Mar-Apr;30(2):239–261. [PMC free article] [PubMed] [Google Scholar]
- SMITH G. T., BEEUWKES R., TOMKIEWICZ M., ABE T., LOWN B. PATHOLOGICAL CHANGES IN SKIN AND SKELETAL MUSCLE FOLLOWING ALTERNATING CURRENT AND CAPACITOR DISCHARGE. Am J Pathol. 1965 Jul;47:1–17. [PMC free article] [PubMed] [Google Scholar]
- Scheinberg M. A., Cathcart E. S., Eastcott J. W., Skinner M., Benson M., Shirahama T. The SJL/J mouse: a new model for spontaneous age-associated amyloidosis. I. Morphologic and immunochemical aspects. Lab Invest. 1976 Jul;35(1):47–54. [PubMed] [Google Scholar]
- Skinner M., Shirahama T., Benson M. D., Cohen A. S. Murine amyloid protein AA in casein-induced experimental amyloidosis. Lab Invest. 1977 Apr;36(4):420–427. [PubMed] [Google Scholar]
- Sorenson G. D., Heefner W. A., Kirkpatrick J. B. Experimental amyloidosis. II. Light and electron microscopic observations of liver. Am J Pathol. 1964 Apr;44(4):629–644. [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Ishikawa S., Motoyama T., Oboshi S. Experimental murine amyloidosis. Evaluation of induction methods and strain difference. Acta Pathol Jpn. 1980 Jul;30(4):549–556. doi: 10.1111/j.1440-1827.1980.tb01350.x. [DOI] [PubMed] [Google Scholar]






