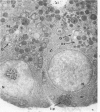
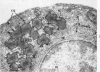
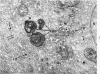
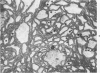
Full text
PDF










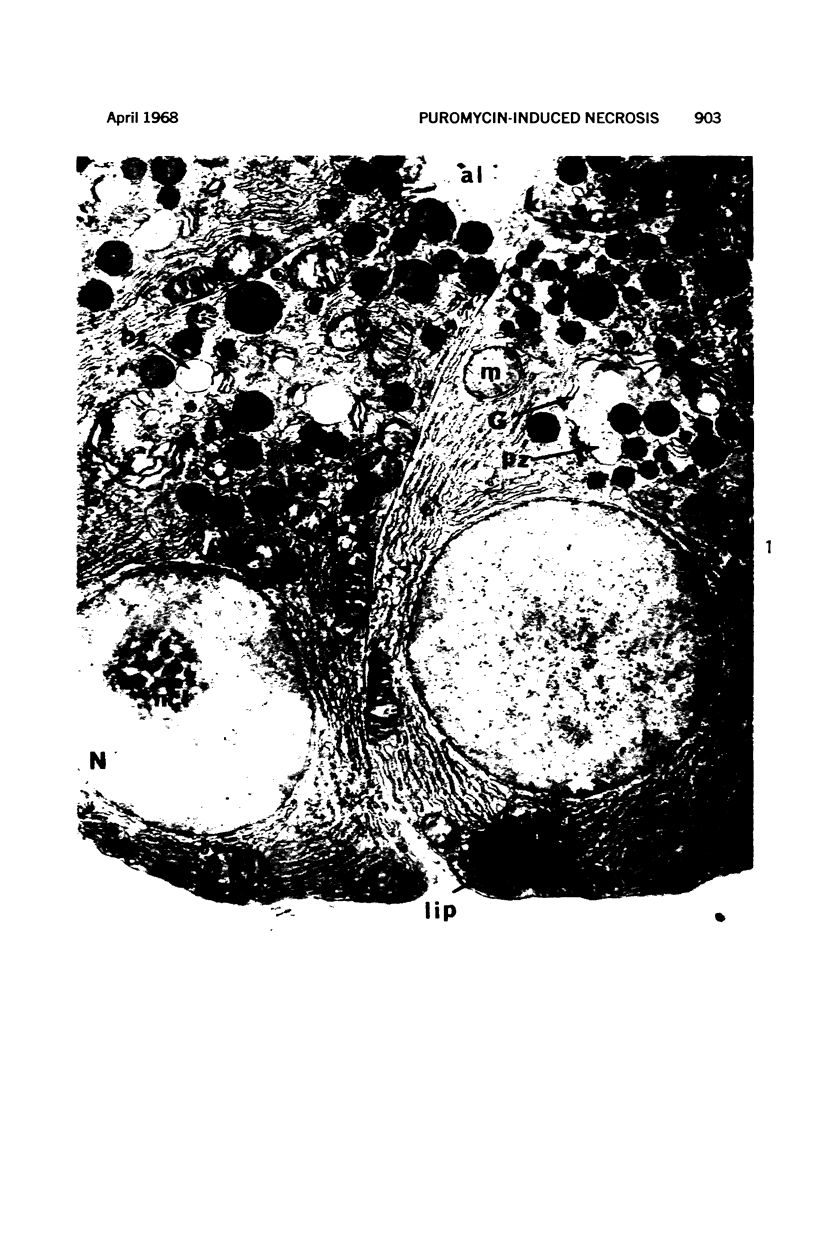

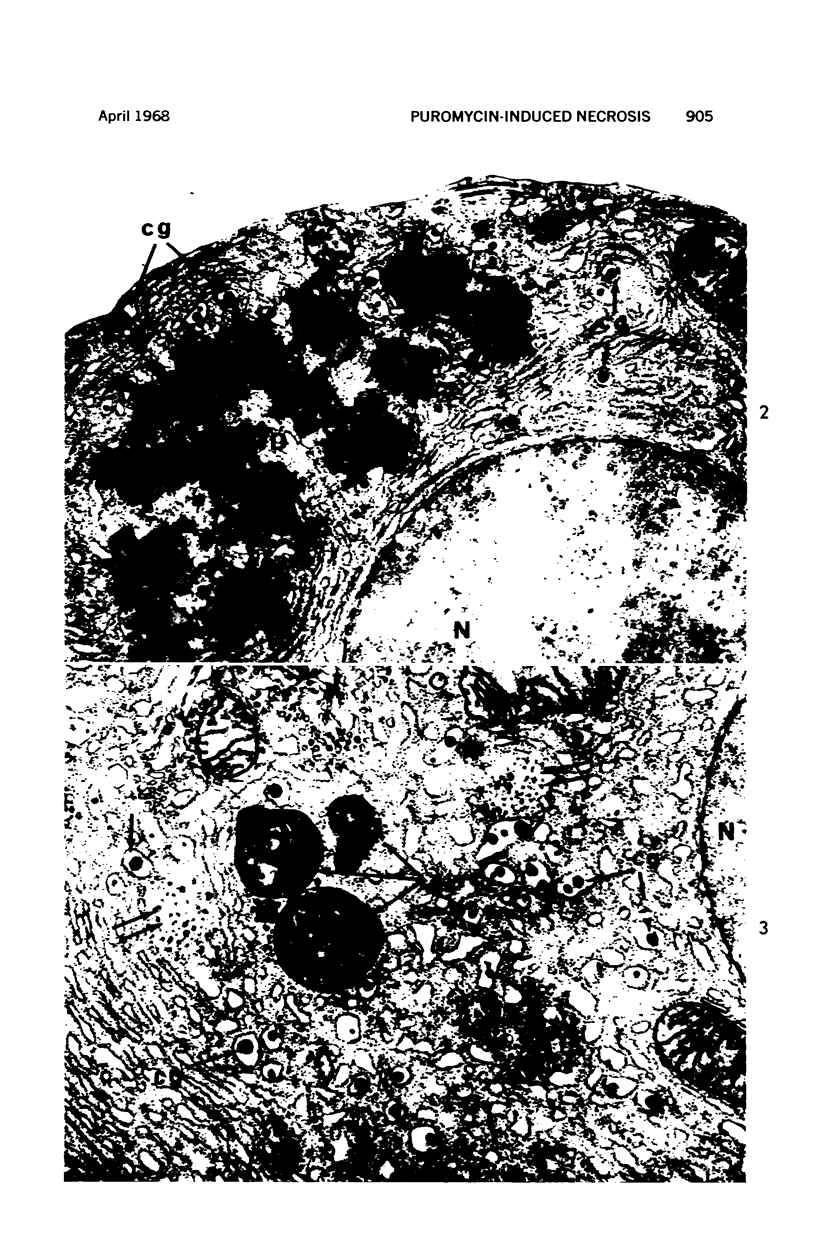
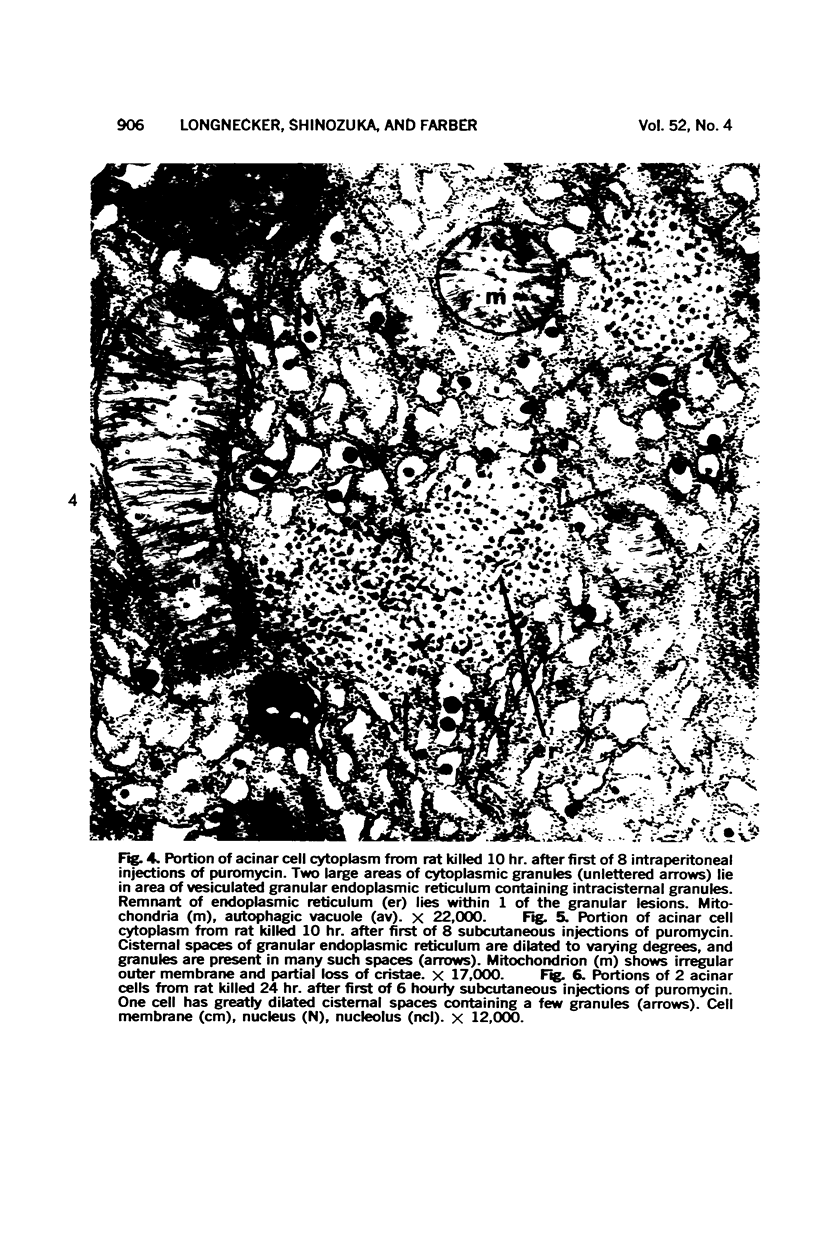
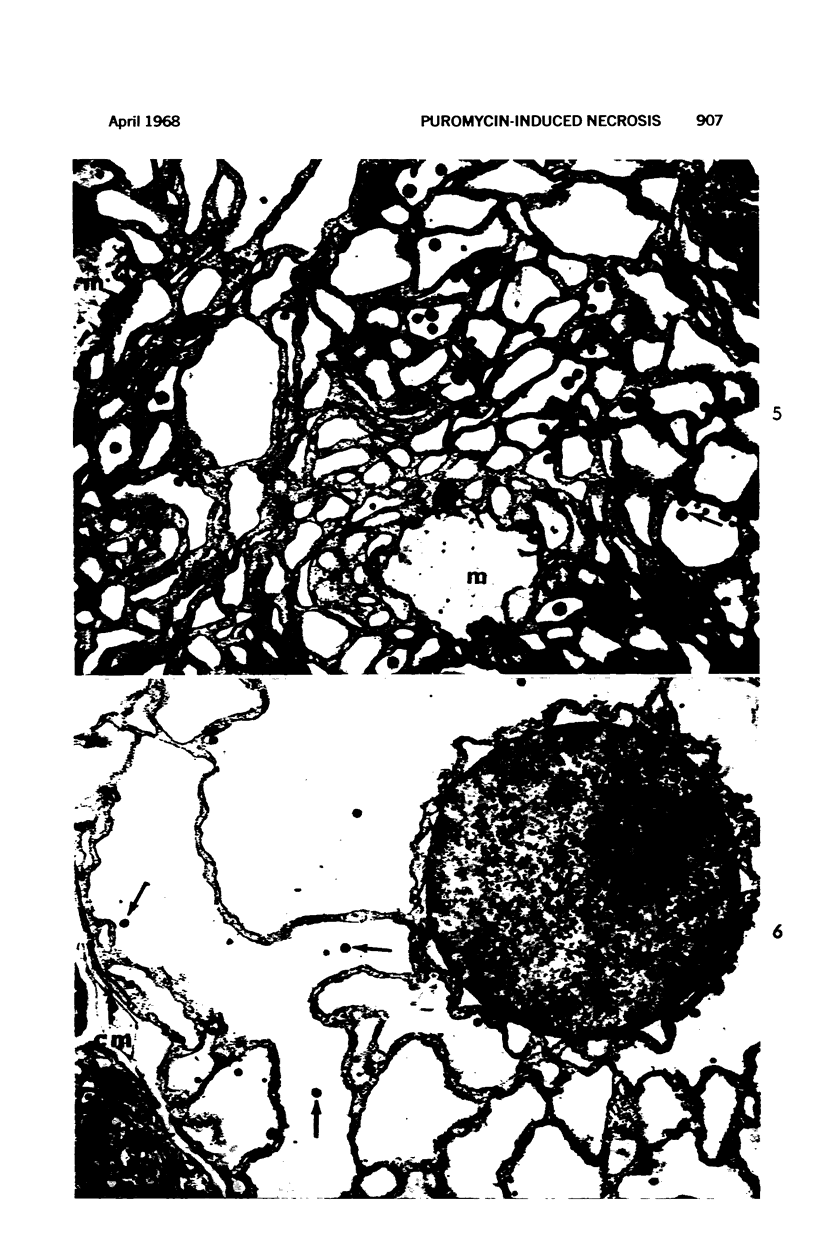

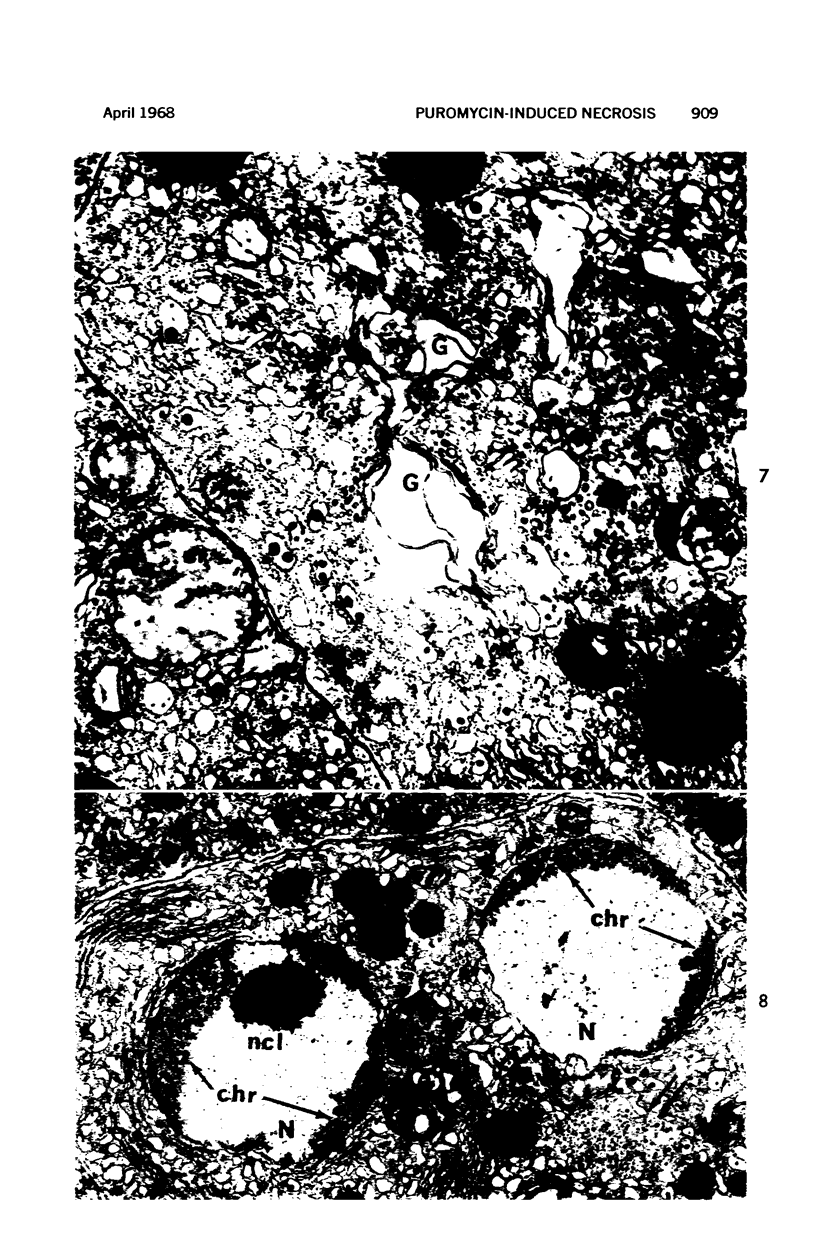

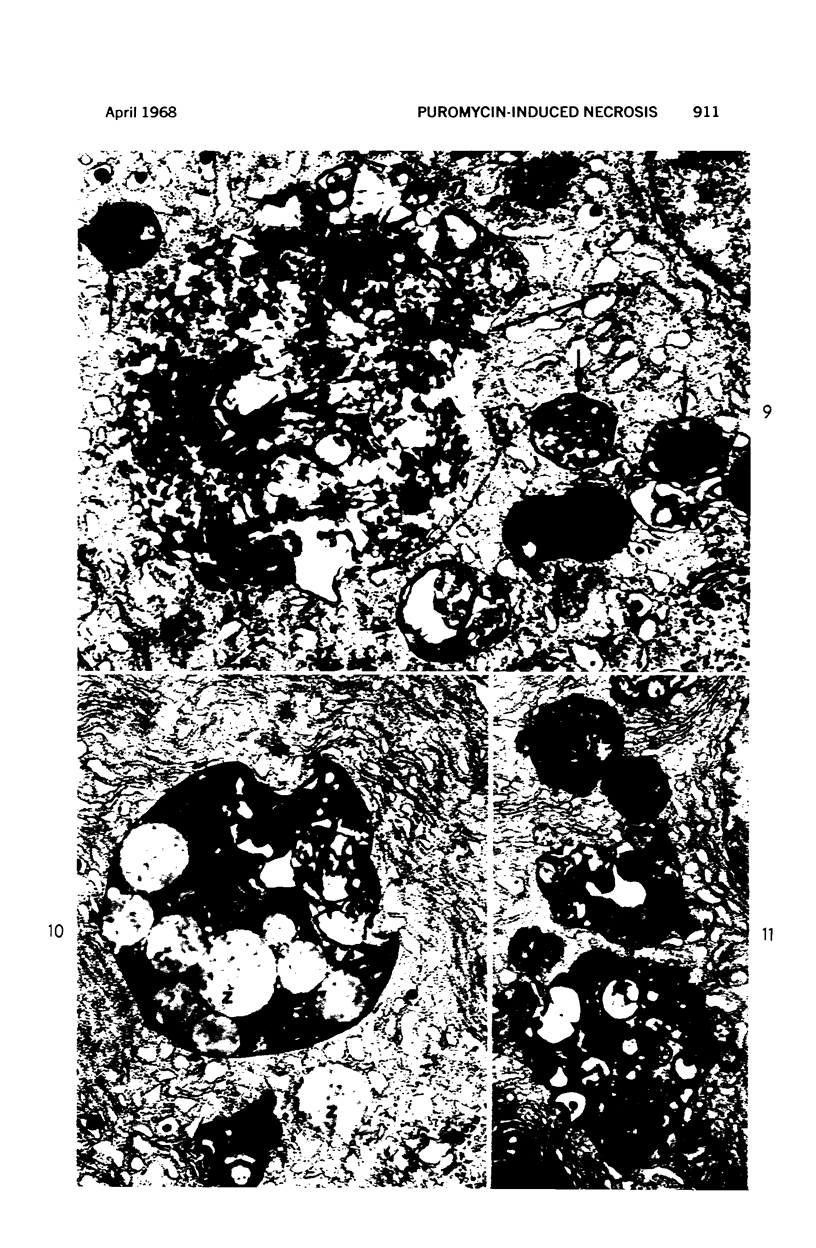

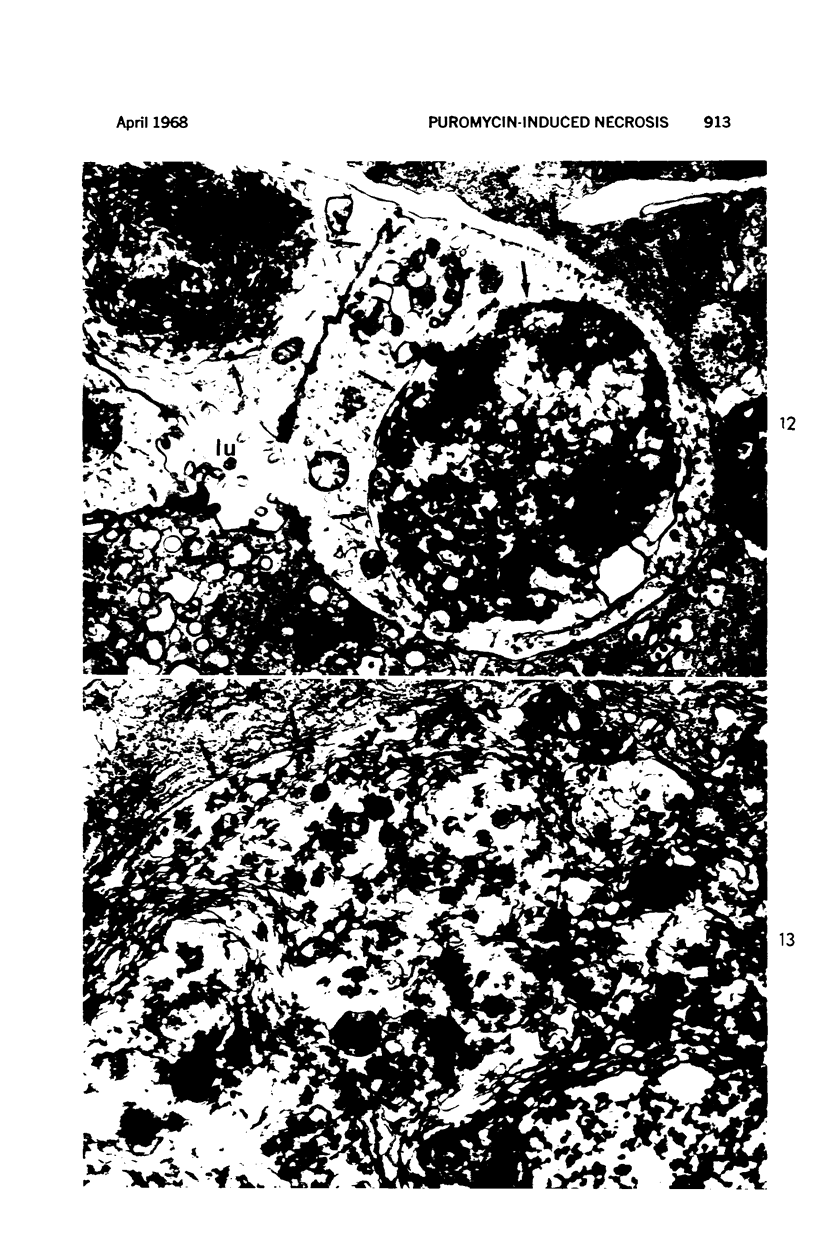

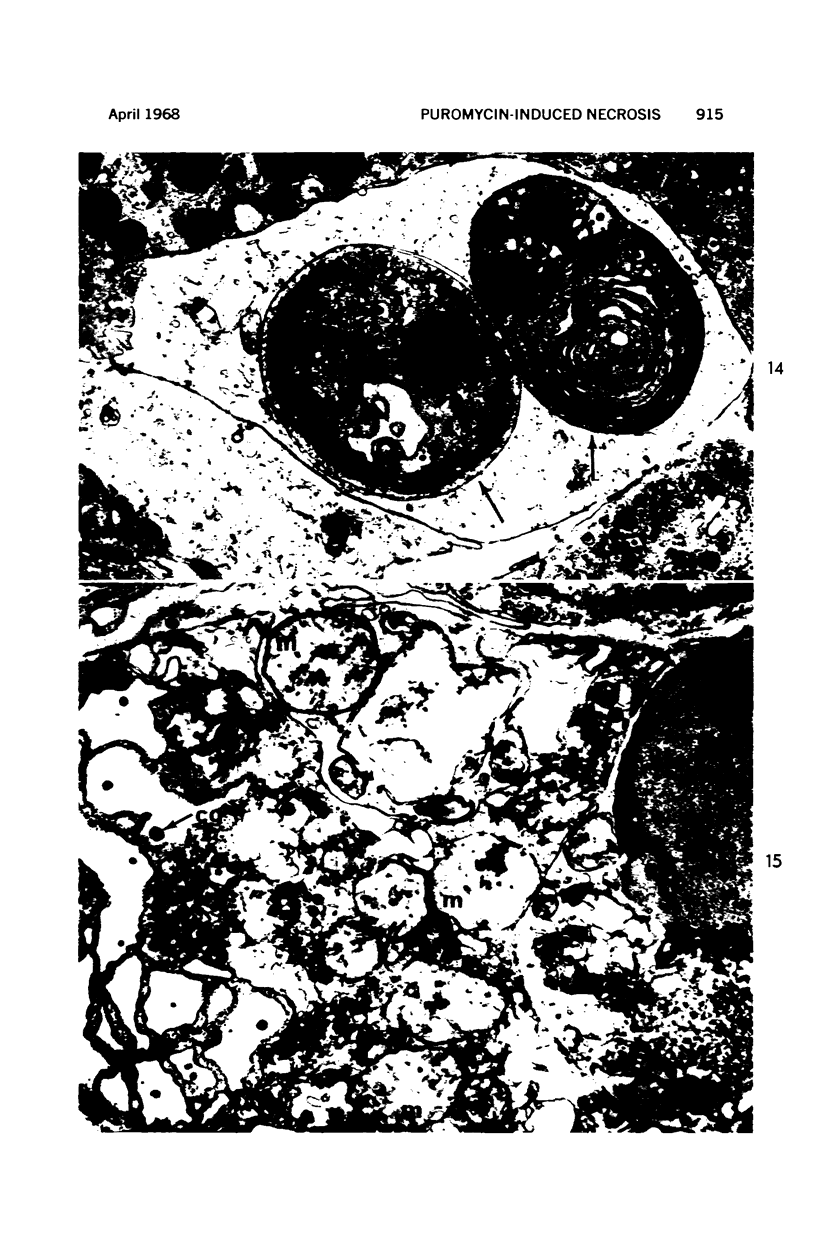
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DARKEN M. A. PUROMYCIN INHIBITION OF PROTEIN SYNTHESIS. Pharmacol Rev. 1964 Sep;16:223–243. [PubMed] [Google Scholar]
- Estensen R. D., Baserga R. Puromycin-induced necrosis of crypt cells of the small intestine of mouse. J Cell Biol. 1966 Jul;30(1):13–22. doi: 10.1083/jcb.30.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMAN L., FITZGERALD P. J. The degenerative changes in pancreatic acinar cells caused by DL-ethionine. J Cell Biol. 1962 Feb;12:277–296. doi: 10.1083/jcb.12.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRUBAN Z., SPARGO B., SWIFT H., WISSLER R. W., KLEINFELD R. G. Focal cytoplasmic degradation. Am J Pathol. 1963 Jun;42:657–683. [PMC free article] [PubMed] [Google Scholar]
- HRUBAN Z., SWIFT H., DUNN F. W., LEWIS D. E. EFFECT OF BETA-3-FURYLALANINE ON THE ULTRASTRUCTURE OF THE HEPATOCYTES AND PANCREATIC ACINAR CELLS. Lab Invest. 1965 Jan;14:70–80. [PubMed] [Google Scholar]
- HRUBAN Z., SWIFT H., WISSLER R. W. Effect of beta-3-thienylalanine on the formation of zymogen granules of exocrine pancreas. J Ultrastruct Res. 1962 Oct;7:359–372. doi: 10.1016/s0022-5320(62)90031-x. [DOI] [PubMed] [Google Scholar]
- HRUBAN Z., VILLA-TREVINO S., FARBER E. EFFECTS OF SOME PHENYLALANINE ANALOGS UPON PROTEIN METABOLISM IN VIVO. Lab Invest. 1965 May;14:468–474. [PubMed] [Google Scholar]
- Longnecker D. S., Farber E. Acute pancreatic necrosis induced by puromycin. Lab Invest. 1967 Mar;16(3):321–329. [PubMed] [Google Scholar]
- PALADE G. E. Intracisternal granules in the exocrine cells of the pancreas. J Biophys Biochem Cytol. 1956 Jul 25;2(4):417–422. doi: 10.1083/jcb.2.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Sabatini D. D. Vectorial discharge of peptides released by puromycin from attached ribosomes. Proc Natl Acad Sci U S A. 1966 Aug;56(2):608–615. doi: 10.1073/pnas.56.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez T. G. Ultrastructural changes in the mouse exocrine pancreas induced by prolonged treatment with actinomycin D. J Ultrastruct Res. 1967 Jul;19(1):116–129. doi: 10.1016/s0022-5320(67)80062-5. [DOI] [PubMed] [Google Scholar]
- SIEKEVITZ P., PALADE G. E. A cytochemical study on the pancreas of the guinea pig. II. Functional variations in the enzymatic activity of microsomes. J Biophys Biochem Cytol. 1958 May 25;4(3):309–318. doi: 10.1083/jcb.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E. B. Histopathology of amino acid deficiencies. Arch Pathol. 1966 Aug;82(2):119–128. [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]
- WEISBLUM B., HERMAN L., FITZGERALD P. J. Changes in pancreatic acinar cells during protein deprivation. J Cell Biol. 1962 Feb;12:313–327. doi: 10.1083/jcb.12.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELANDER T., EKHOLM R., EDLUND Y. The ultrastructural organization of the rat exocrine pancreas. III. Intralobular vessels and nerves. J Ultrastruct Res. 1962 Aug;7:84–101. doi: 10.1016/s0022-5320(62)80029-x. [DOI] [PubMed] [Google Scholar]