Abstract
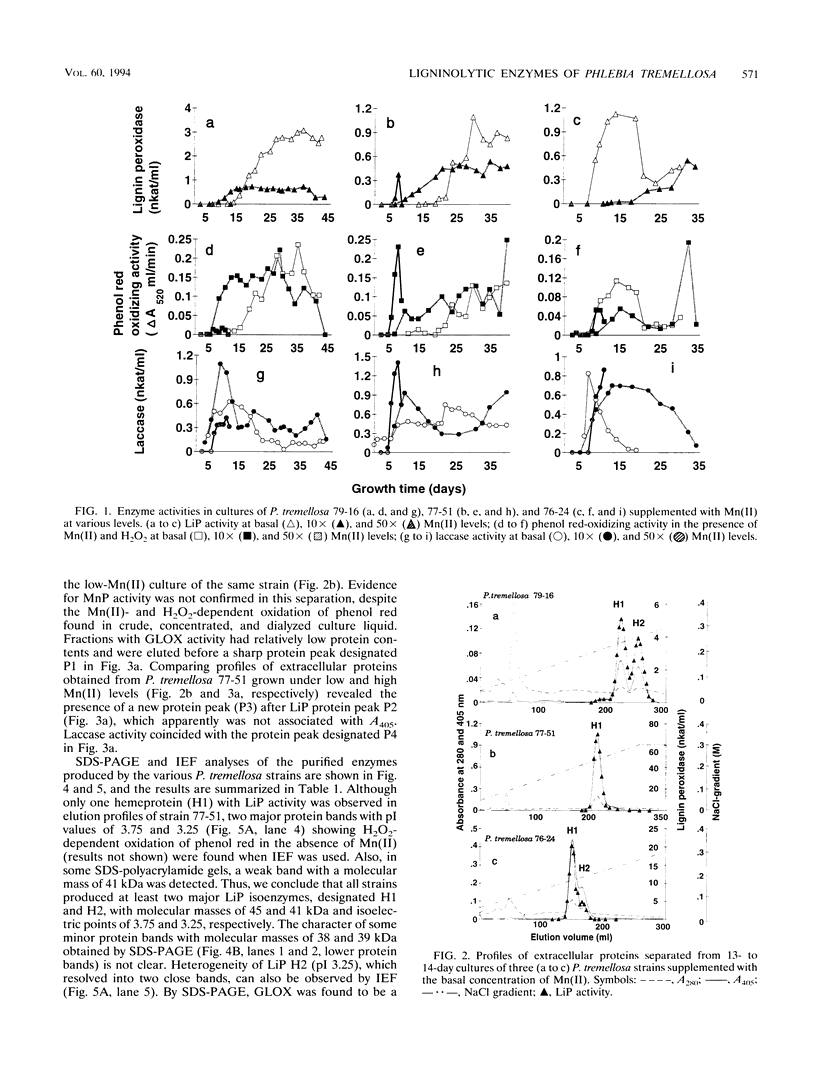
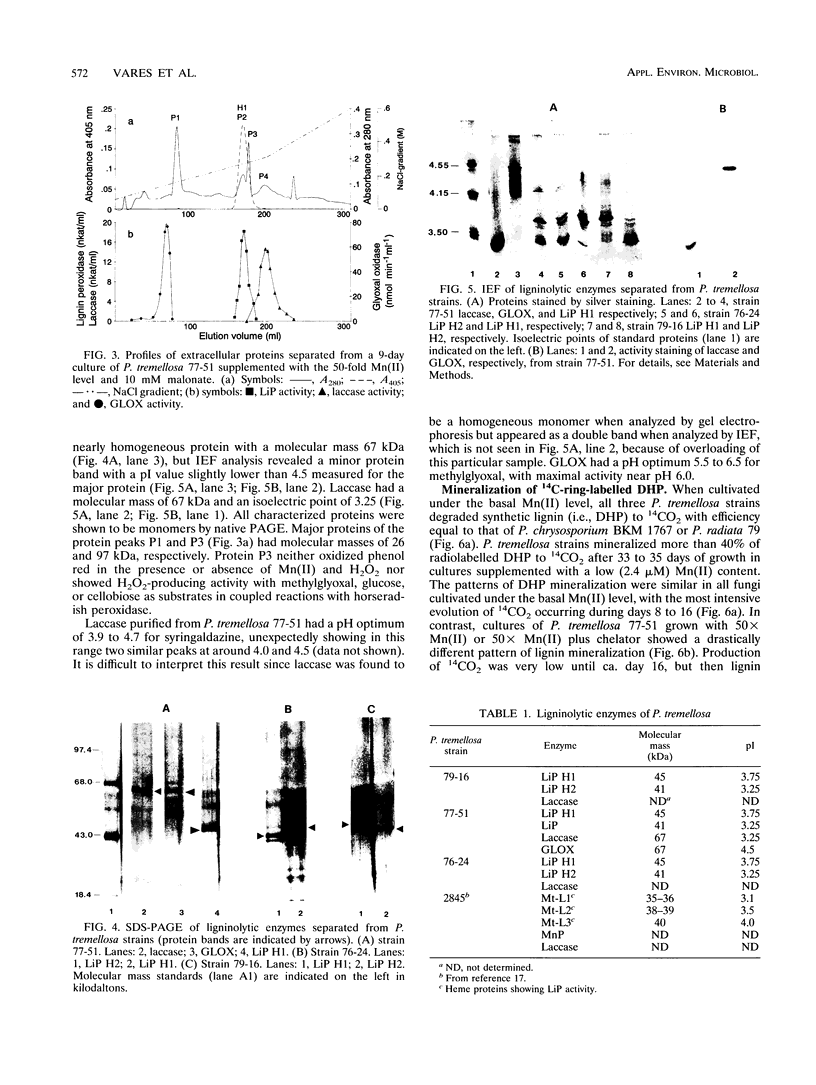
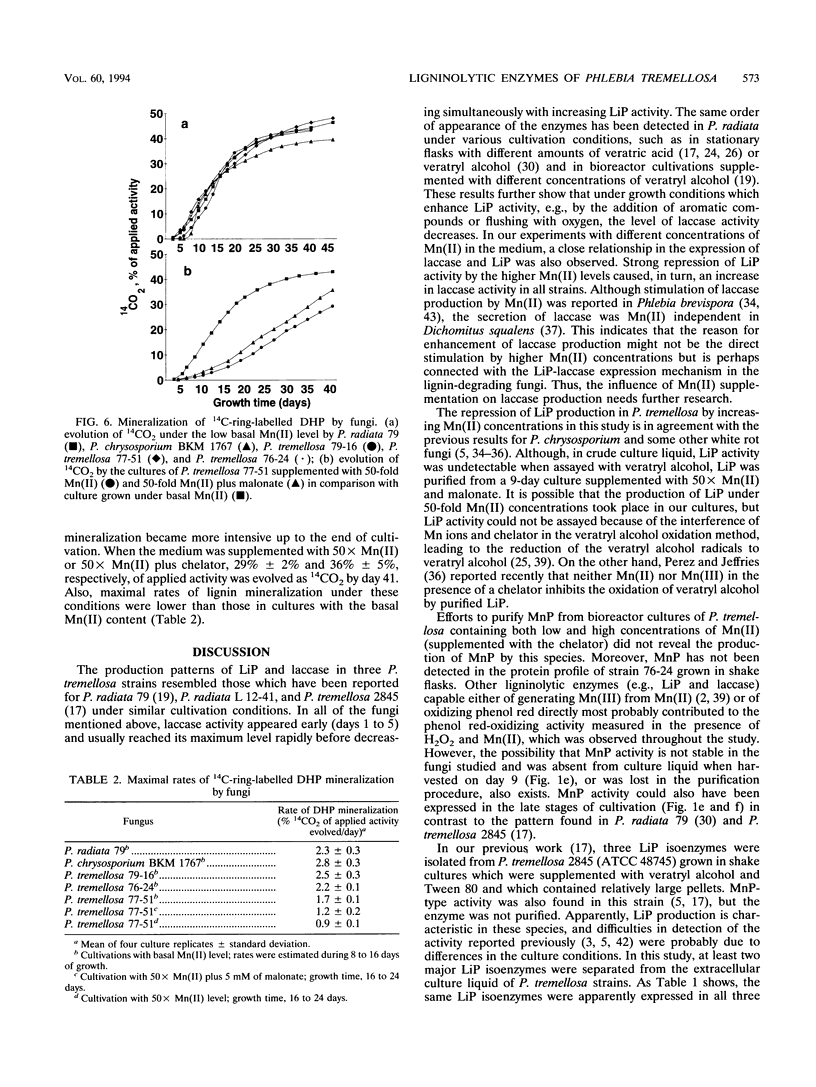
Production of ligninolytic enzymes by three strains of the white rot fungus Phlebia tremellosa (syn. Merulius tremellosus) was studied in bioreactor cultivation under nitrogen-limiting conditions. The Mn(II) concentration of the growth medium strongly affected the secretion patterns of lignin peroxidase and laccase. Two major lignin peroxidase isoenzymes were expressed in all strains. In addition, laccase and glyoxal oxidase were purified and characterized in one strain of P. tremellosa. In contrast, manganese peroxidase was not found in fast protein liquid chromatography profiles of extracellular proteins under either low (2.4 μM) or elevated (24 and 120 μM) Mn(II) concentrations. However, H2O2- and Mn-dependent phenol red-oxidizing activity was detected in cultures supplemented with higher Mn(II) levels. Mineralization rates of 14C-ring-labelled synthetic lignin (i.e., dehydrogenation polymerizate) by all strains under a low basal Mn(II) level were similar to those obtained for Phanerochaete chrysosporium and Phlebia radiata. A high manganese concentration repressed the evolution of 14CO2 even when a chelating agent, sodium malonate, was included in the medium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F., Roy B. Production of manganic chelates by laccase from the lignin-degrading fungus Trametes (Coriolus) versicolor. Appl Environ Microbiol. 1992 May;58(5):1496–1499. doi: 10.1128/aem.58.5.1496-1499.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette R. A., Reid I. D. Ultrastructural Aspects of Wood Delignification by Phlebia (Merulius) tremellosus. Appl Environ Microbiol. 1986 Aug;52(2):239–245. doi: 10.1128/aem.52.2.239-245.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnarme P., Jeffries T. W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl Environ Microbiol. 1990 Jan;56(1):210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. A., Glenn J. K., Gold M. H. Manganese regulates expression of manganese peroxidase by Phanerochaete chrysosporium. J Bacteriol. 1990 Jun;172(6):3125–3130. doi: 10.1128/jb.172.6.3125-3130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel K. E., Jensen K. A., Jr, Mozuch M. D., Landucci L. L., Tien M., Pease E. A. Ligninolysis by a purified lignin peroxidase. J Biol Chem. 1993 Jun 15;268(17):12274–12281. [PubMed] [Google Scholar]
- Kersten P. J. Glyoxal oxidase of Phanerochaete chrysosporium: its characterization and activation by lignin peroxidase. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2936–2940. doi: 10.1073/pnas.87.8.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Kirk T. K. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J Bacteriol. 1987 May;169(5):2195–2201. doi: 10.1128/jb.169.5.2195-2201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundell T., Leonowicz A., Rogalski J., Hatakka A. Formation and Action of Lignin-Modifying Enzymes in Cultures of Phlebia radiata Supplemented with Veratric Acid. Appl Environ Microbiol. 1990 Sep;56(9):2623–2629. doi: 10.1128/aem.56.9.2623-2629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niku-Paavola M. L., Karhunen E., Salola P., Raunio V. Ligninolytic enzymes of the white-rot fungus Phlebia radiata. Biochem J. 1988 Sep 15;254(3):877–883. doi: 10.1042/bj2540877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease E. A., Aust S. D., Tien M. Heterologous expression of active manganese peroxidase from Phanerochaete chrysosporium using the baculovirus expression system. Biochem Biophys Res Commun. 1991 Sep 16;179(2):897–903. doi: 10.1016/0006-291x(91)91903-p. [DOI] [PubMed] [Google Scholar]
- Perez J., Jeffries T. W. Mineralization of C-Ring-Labeled Synthetic Lignin Correlates with the Production of Lignin Peroxidase, not of Manganese Peroxidase or Laccase. Appl Environ Microbiol. 1990 Jun;56(6):1806–1812. doi: 10.1128/aem.56.6.1806-1812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J., Jeffries T. W. Role of organic acid chelators in manganese regulation of lignin degradation by Phanerochaete chrysosporium. Appl Biochem Biotechnol. 1993 Spring;39-40:227–238. doi: 10.1007/BF02918992. [DOI] [PubMed] [Google Scholar]
- Perez J., Jeffries T. W. Roles of manganese and organic acid chelators in regulating lignin degradation and biosynthesis of peroxidases by Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Aug;58(8):2402–2409. doi: 10.1128/aem.58.8.2402-2409.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C. R., Matcham S. E., Wood D. A., Thurston C. F. The structure of laccase protein and its synthesis by the commercial mushroom Agaricus bisporus. J Gen Microbiol. 1993 Jan;139(1):171–178. doi: 10.1099/00221287-139-1-171. [DOI] [PubMed] [Google Scholar]
- Popp J. L., Kalyanaraman B., Kirk T. K. Lignin peroxidase oxidation of Mn2+ in the presence of veratryl alcohol, malonic or oxalic acid, and oxygen. Biochemistry. 1990 Nov 20;29(46):10475–10480. doi: 10.1021/bi00498a008. [DOI] [PubMed] [Google Scholar]
- Périé F. H., Gold M. H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl Environ Microbiol. 1991 Aug;57(8):2240–2245. doi: 10.1128/aem.57.8.2240-2245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid I. D. Biological Delignification of Aspen Wood by Solid-State Fermentation with the White-Rot Fungus Merulius tremellosus. Appl Environ Microbiol. 1985 Jul;50(1):133–139. doi: 10.1128/aem.50.1.133-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]




