Abstract
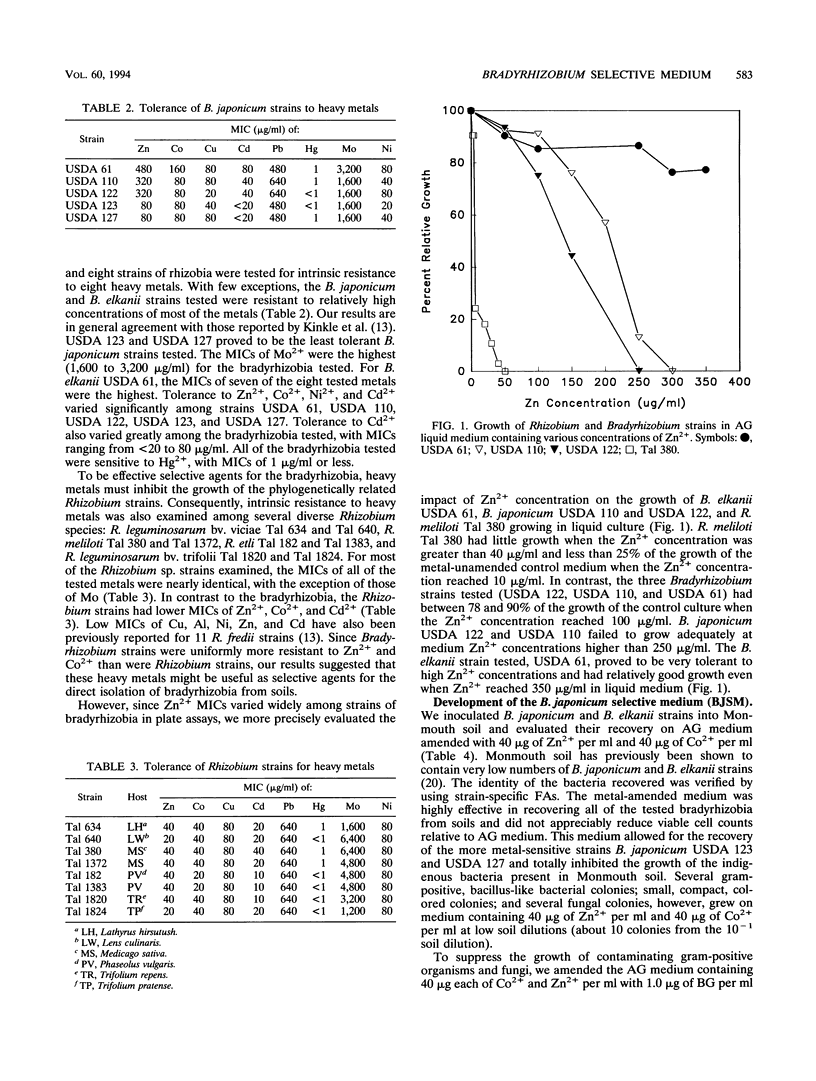
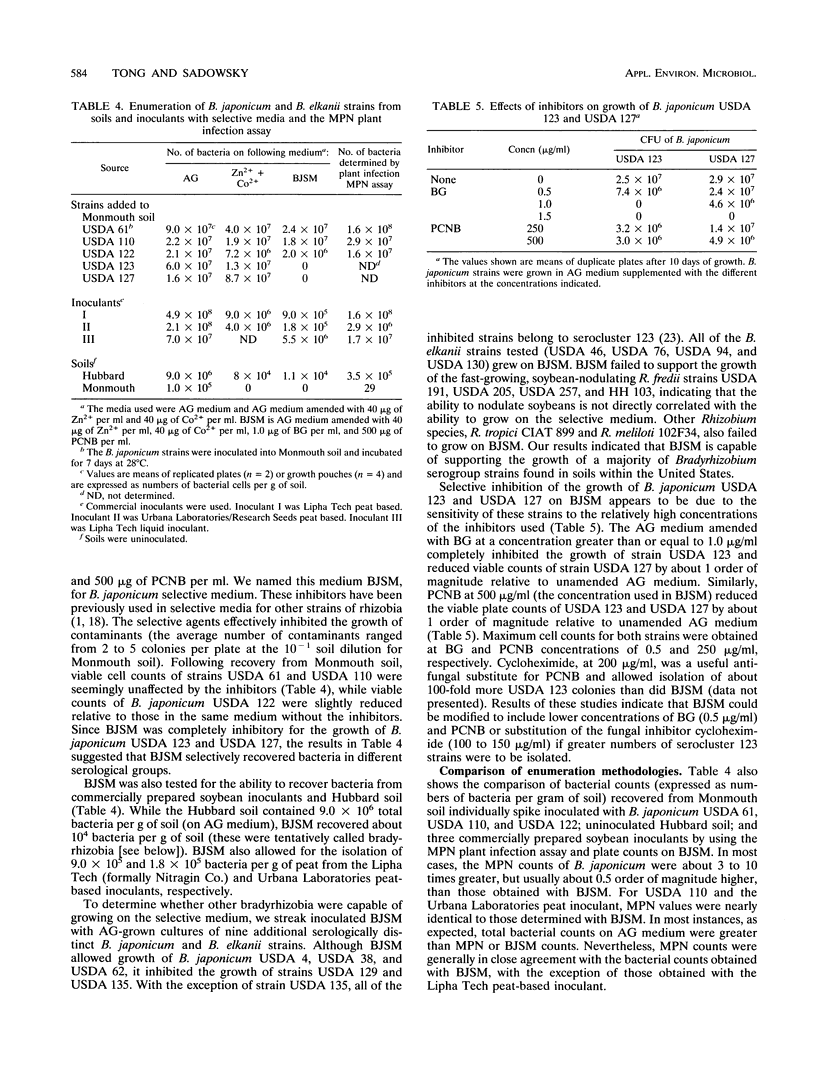
The ecological examination of members of the family Rhizobiaceae has been hampered by the lack of a selective medium for isolation of root nodule bacteria from soil. A novel non-antibiotic-containing medium has been developed which allows selective isolation of Bradyrhizobium japonicum and B. elkanii strains from soil and inoculants. The medium, BJSM, is based on the resistance of B.japonicum and B. elkanii strains to more than 40 μg of the metals ions Zn2+ and Co2+ per ml. BJSM does not allow growth of Rhizobium sp. strains. We used BJSM to isolate bacteria from a Hubbard soil and from several commercially prepared soybean inoculants. Ninety-eight percent of the isolates obtained from Hubbard soil nodulated Glycine max cv. Kasota, and between 55 and 95% of the isolates from the commercial inoculants had the ability to nodulate soybeans. Numbers of bradyrhizobia obtained by using BJSM, strain-specific fluorescent antibodies, and the most-probable-number plant infection assay indicated that the three techniques were comparable in quantifying B. japonicum strains in soils and inoculants, although most-probable-number counts were generally 0.5 order of magnitude greater than those obtained by using BJSM. Results of our studies indicate that BJSM is useful for direct isolation and quantification of B. japonicum and B. elkanii from natural soils and inoculants. This medium may prove to be an important tool for autecological and enumeration studies of diverse populations of bradyrhizobia and as a quality control method for soybean inoculants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brynhildsen L., Rosswall T. Effects of cadmium, copper, magnesium, and zinc on the decomposition of citrate by a Klebsiella sp. Appl Environ Microbiol. 1989 Jun;55(6):1375–1379. doi: 10.1128/aem.55.6.1375-1379.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danso S. K., Habte M., Alexander M. Estimating the density of individual bacterial populations introduced into natural ecosytems. Can J Microbiol. 1973 Nov;19(11):1450–1451. doi: 10.1139/m73-234. [DOI] [PubMed] [Google Scholar]
- Dean-Ross Deborah, Mills Aaron L. Bacterial Community Structure and Function along a Heavy Metal Gradient. Appl Environ Microbiol. 1989 Aug;55(8):2002–2009. doi: 10.1128/aem.55.8.2002-2009.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diels L., Mergeay M. DNA probe-mediated detection of resistant bacteria from soils highly polluted by heavy metals. Appl Environ Microbiol. 1990 May;56(5):1485–1491. doi: 10.1128/aem.56.5.1485-1491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis A. J., Dodge C. J. Anaerobic microbial dissolution of transition and heavy metal oxides. Appl Environ Microbiol. 1988 Apr;54(4):1009–1014. doi: 10.1128/aem.54.4.1009-1014.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham P. H. Selective medium for growth of Rhizobium. Appl Microbiol. 1969 May;17(5):769–770. doi: 10.1128/am.17.5.769-770.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansson M., Jones G. W., Kjelleberg S. Frequency of antibiotic and heavy metal resistance, pigmentation, and plasmids in bacteria of the marine air-water interface. Appl Environ Microbiol. 1987 Oct;53(10):2338–2342. doi: 10.1128/aem.53.10.2338-2342.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley M. T., Bohlool B. B. Release of Rhizobium spp. from Tropical Soils and Recovery for Immunofluorescence Enumeration. Appl Environ Microbiol. 1981 Aug;42(2):241–248. doi: 10.1128/aem.42.2.241-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkle B. K., Angle J. S., Keyser H. H. Long-Term Effects of Metal-Rich Sewage Sludge Application on Soil Populations of Bradyrhizobium japonicum. Appl Environ Microbiol. 1987 Feb;53(2):315–319. doi: 10.1128/aem.53.2.315-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies D. H., Nies A., Chu L., Silver S. Expression and nucleotide sequence of a plasmid-determined divalent cation efflux system from Alcaligenes eutrophus. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7351–7355. doi: 10.1073/pnas.86.19.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies D. H., Silver S. Metal ion uptake by a plasmid-free metal-sensitive Alcaligenes eutrophus strain. J Bacteriol. 1989 Jul;171(7):4073–4075. doi: 10.1128/jb.171.7.4073-4075.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies D. H., Silver S. Plasmid-determined inducible efflux is responsible for resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus. J Bacteriol. 1989 Feb;171(2):896–900. doi: 10.1128/jb.171.2.896-900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies D., Mergeay M., Friedrich B., Schlegel H. G. Cloning of plasmid genes encoding resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus CH34. J Bacteriol. 1987 Oct;169(10):4865–4868. doi: 10.1128/jb.169.10.4865-4868.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison A. C., Skinner F. A. The effects of antimicrobial substances on Rhizobium spp. and their use in selective media. J Appl Bacteriol. 1974 Jun;37(2):239–250. doi: 10.1111/j.1365-2672.1974.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Sadowsky M. J., Tully R. E., Cregan P. B., Keyser H. H. Genetic Diversity in Bradyrhizobium japonicum Serogroup 123 and Its Relation to Genotype-Specific Nodulation of Soybean. Appl Environ Microbiol. 1987 Nov;53(11):2624–2630. doi: 10.1128/aem.53.11.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Bakole R. O., Bohlool B. B. Fluorescent-antibody approach to study of rhizobia in soil. J Bacteriol. 1968 Jun;95(6):1987–1992. doi: 10.1128/jb.95.6.1987-1992.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Zidwick M. J., Abebe H. M. Bradyrhizobium japonicum Serocluster 123 and Diversity among Member Isolates. Appl Environ Microbiol. 1986 Jun;51(6):1212–1215. doi: 10.1128/aem.51.6.1212-1215.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia L., Piñero D., Palacios R., Martínez-Romero E. Genetic structure of a soil population of nonsymbiotic Rhizobium leguminosarum. Appl Environ Microbiol. 1991 Feb;57(2):426–433. doi: 10.1128/aem.57.2.426-433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Misra T. K. Plasmid-mediated heavy metal resistances. Annu Rev Microbiol. 1988;42:717–743. doi: 10.1146/annurev.mi.42.100188.003441. [DOI] [PubMed] [Google Scholar]
- Top E., Mergeay M., Springael D., Verstraete W. Gene escape model: transfer of heavy metal resistance genes from Escherichia coli to Alcaligenes eutrophus on agar plates and in soil samples. Appl Environ Microbiol. 1990 Aug;56(8):2471–2479. doi: 10.1128/aem.56.8.2471-2479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woomer P. L., Singleton P. W., Bohlool B. B. Reliability of the most-probable-number technique for enumerating rhizobia in tropical soils. Appl Environ Microbiol. 1988 Jun;54(6):1494–1497. doi: 10.1128/aem.54.6.1494-1497.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


