Abstract
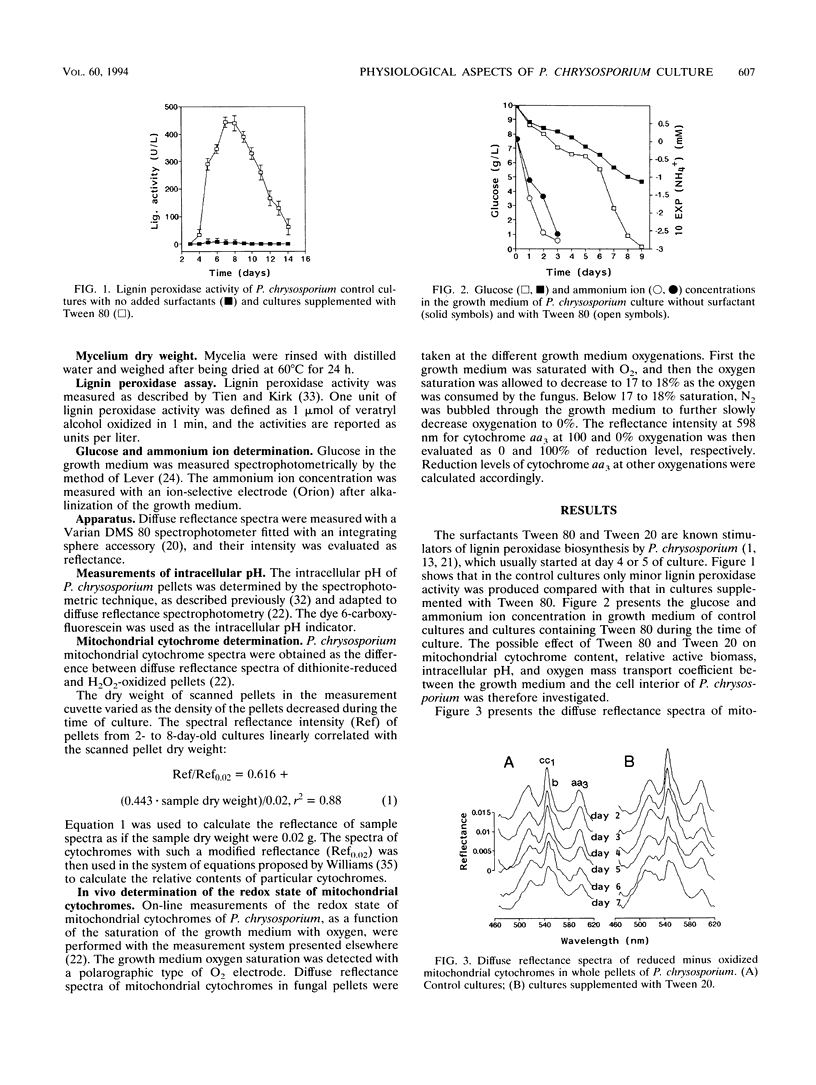
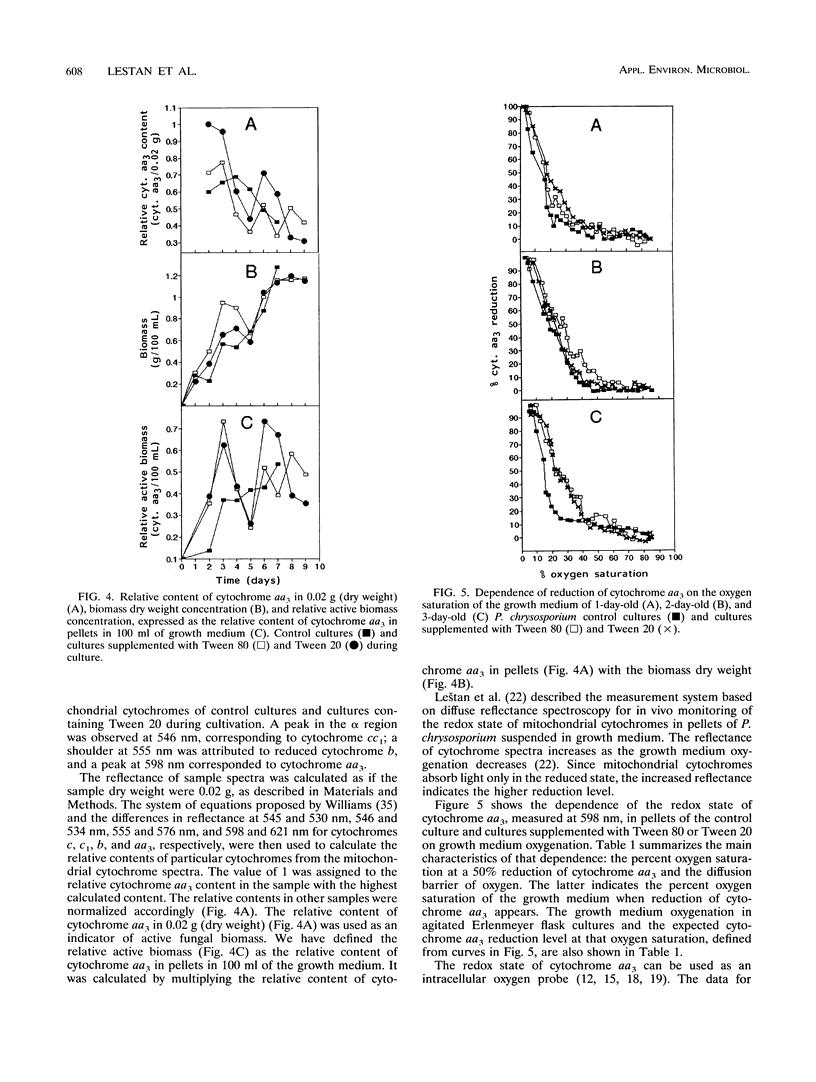
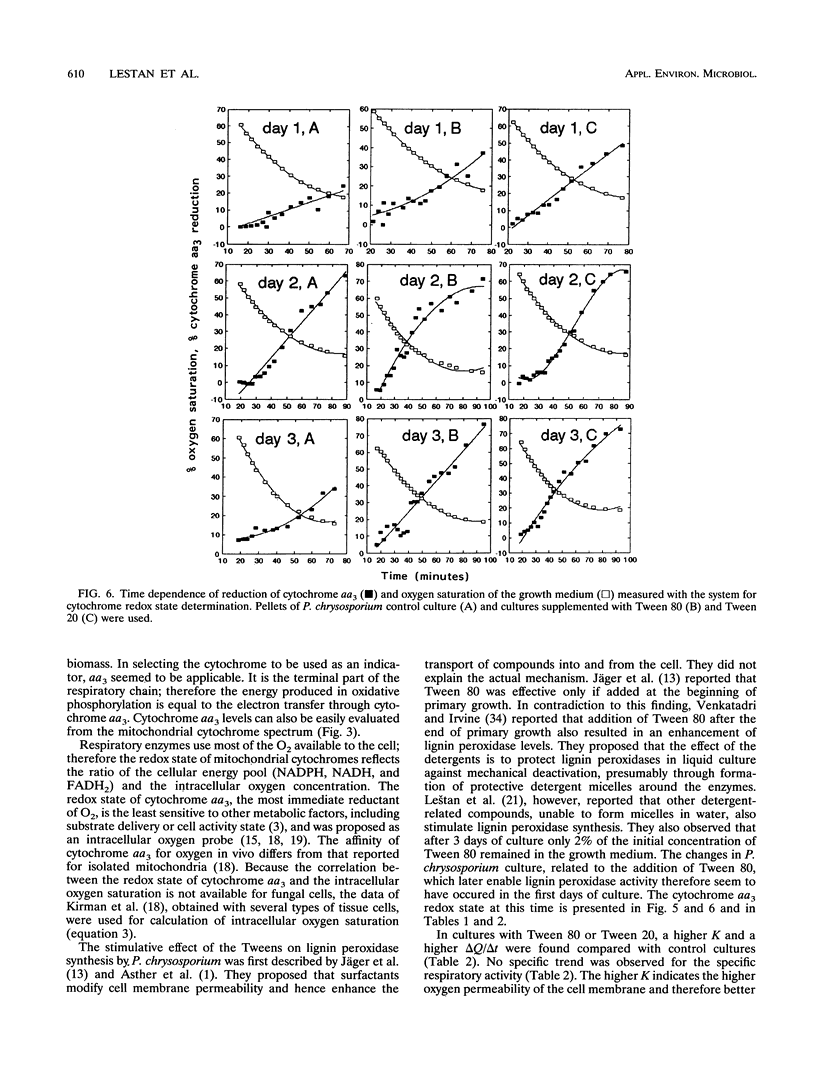
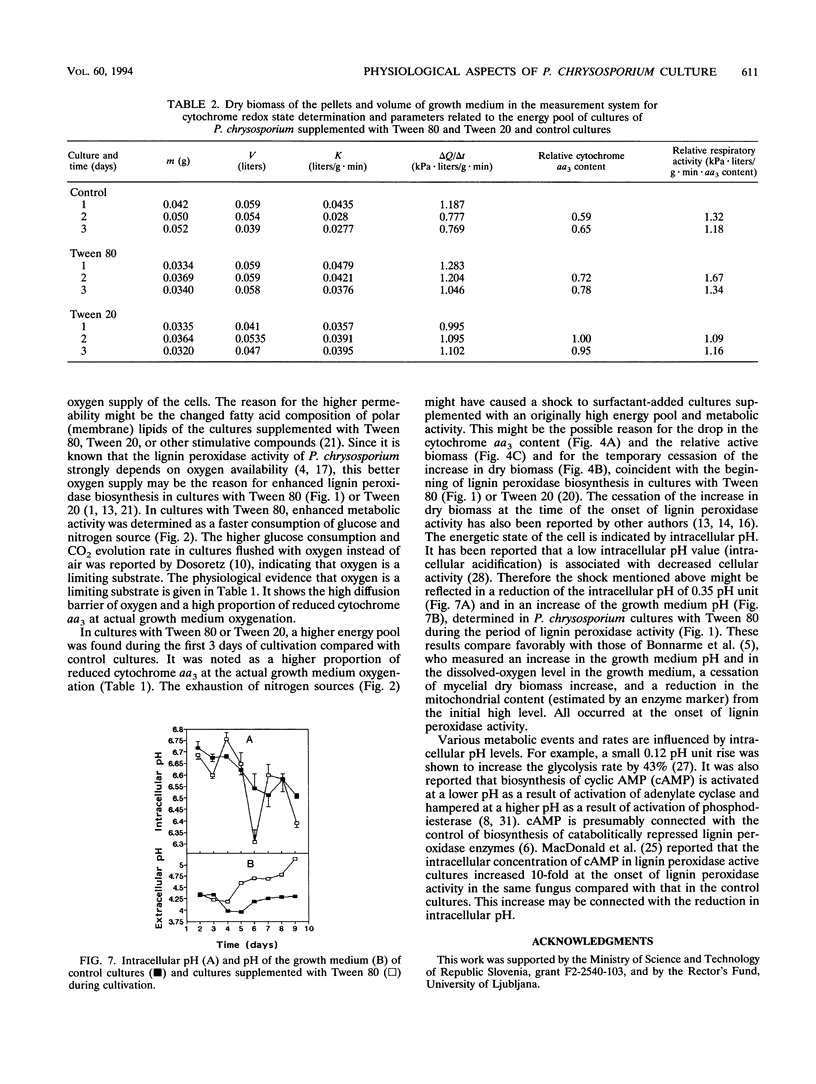
Methods based on UV-visible diffuse reflectance spectroscopy were used to study the physiological aspects of lignin-peroxidase biosynthesis by Phanerochaete chrysosporium. Here we introduce the use of cytochrome aa3 as an indicator of active fungal biomass and of its redox state to calculate the oxygen mass transport coefficient between the growth medium and the fungal cell interior. When lignin peroxidase biosynthesis was enhanced by the addition of Tween 80 or Tween 20 to the growth medium, a higher proportion of reduced cytochrome aa3 and a higher oxygen diffusion barrier were observed compared with control cultures. In cultures supplemented with Tween 80 or Tween 20, a higher oxygen mass transport coefficient between the growth medium and the interior of the fungal cell was also found. The beginning of the lignin peroxidase activity in these cultures was found to coincide with a temporary cessation in the dry biomass increase and a reduction in the relative active-biomass concentration. During the lignin peroxidase activity, a decrease in the intracellular pH and an increase in the growth medium pH were determined in cultures supplemented with Tween 80.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Lev S. S., Kirk T. K. Effects of molecular oxygen on lignin degradation by Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1981 Mar 31;99(2):373–378. doi: 10.1016/0006-291x(81)91755-1. [DOI] [PubMed] [Google Scholar]
- Broda P., Sims P. F., Mason J. C. Lignin biodegradation: a molecular biological approach. Essays Biochem. 1989;24:82–114. [PubMed] [Google Scholar]
- Bumpus J. A., Tien M., Wright D., Aust S. D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985 Jun 21;228(4706):1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984 Apr;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Capdevila C., Moukha S., Ghyczy M., Theilleux J., Gelie B., Delattre M., Corrieu G., Asther M. Characterization of Peroxidase Secretion and Subcellular Organization of Phanerochaete chrysosporium INA-12 in the Presence of Various Soybean Phospholipid Fractions. Appl Environ Microbiol. 1990 Dec;56(12):3811–3816. doi: 10.1128/aem.56.12.3811-3816.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich U., Hoffmann J., Lübbers D. W. Quantitative evaluation of optical reflection spectra of blood-free perfused guinea pig brain using a nonlinear multicomponent analysis. Pflugers Arch. 1987 Jun;409(1-2):152–157. doi: 10.1007/BF00584764. [DOI] [PubMed] [Google Scholar]
- Jeffries T. W., Choi S., Kirk T. K. Nutritional Regulation of Lignin Degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1981 Aug;42(2):290–296. doi: 10.1128/aem.42.2.290-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger A., Croan S., Kirk T. K. Production of Ligninases and Degradation of Lignin in Agitated Submerged Cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Nov;50(5):1274–1278. doi: 10.1128/aem.50.5.1274-1278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis F. F., Keizer J. H., LaManna J. C., Rosenthal M. Reflectance spectrophotometry of cytochrome aa3 in vivo. J Appl Physiol Respir Environ Exerc Physiol. 1977 Nov;43(5):858–872. doi: 10.1152/jappl.1977.43.5.858. [DOI] [PubMed] [Google Scholar]
- Kariman K., Hempel F. G., Jöbsis F. F., Burns S. R., Saltzman H. A. In vivo comparison of cerebral tissue PO2 and cytochrome aa3 reduction-oxidation state in cats during hemorrhagic shock. J Clin Invest. 1981 Jul;68(1):21–27. doi: 10.1172/JCI110237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariman K., Hempel F. G., Jöbsis F. F. In vivo comparison of cytochrome aa3 redox state and tissue PO2 in transient anoxia. J Appl Physiol Respir Environ Exerc Physiol. 1983 Oct;55(4):1057–1063. doi: 10.1152/jappl.1983.55.4.1057. [DOI] [PubMed] [Google Scholar]
- Keyser P., Kirk T. K., Zeikus J. G. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978 Sep;135(3):790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestan D., Podgornik H., Perdih A. Analysis of Fungal Pellets by UV-Visible Spectrum Diffuse Reflectance Spectroscopy. Appl Environ Microbiol. 1993 Dec;59(12):4253–4260. doi: 10.1128/aem.59.12.4253-4260.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M. A new reaction for colorimetric determination of carbohydrates. Anal Biochem. 1972 May;47(1):273–279. doi: 10.1016/0003-2697(72)90301-6. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J., Paterson A., Broda P. Possible relationship between cyclic AMP and idiophasic metabolism in the white rot fungus Phanerochaete chrysosporium. J Bacteriol. 1984 Oct;160(1):470–472. doi: 10.1128/jb.160.1.470-472.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F. C., Jr, Grulke E. A., Reddy C. A. Determination of the respiration kinetics for mycelial pellets of Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 May;58(5):1740–1745. doi: 10.1128/aem.58.5.1740-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese E. T., Maguire A. Surfactants as stimulants of enzyme production by microorganisms. Appl Microbiol. 1969 Feb;17(2):242–245. doi: 10.1128/am.17.2.242-245.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M., Beullens M., Honshoven F., Hoebeeck G., Detremerie K., den Hollander J. A., Jans A. W. Regulation of the cAMP level in the yeast Saccharomyces cerevisiae: intracellular pH and the effect of membrane depolarizing compounds. J Gen Microbiol. 1987 Aug;133(8):2191–2196. doi: 10.1099/00221287-133-8-2191. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatadri R., Irvine R. L. Effect of Agitation on Ligninase Activity and Ligninase Production by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Sep;56(9):2684–2691. doi: 10.1128/aem.56.9.2684-2691.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS J. N., Jr A METHOD FOR THE SIMULTANEOUS QUANTITATIVE ESTIMATION OF CYTOCHROMES A, B, C1, AND C IN MITOCHONDRIA. Arch Biochem Biophys. 1964 Sep;107:537–543. doi: 10.1016/0003-9861(64)90313-3. [DOI] [PubMed] [Google Scholar]
- Zabriskie D. W., Humphrey A. E. Estimation of fermentation biomass concentration by measuring culture fluorescence. Appl Environ Microbiol. 1978 Feb;35(2):337–343. doi: 10.1128/aem.35.2.337-343.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


