Abstract
Bipolar spindles assemble in the absence of centrosomes in the oocytes of many species. In Drosophila melanogaster oocytes, the chromosomes have been proposed to initiate spindle assembly by nucleating or capturing microtubules, although the mechanism is not understood. An important contributor to this process is Subito, which is a kinesin-6 protein that is required for bundling interpolar microtubules located within the central spindle at metaphase I. We have characterized the domains of Subito that regulate its activity and its specificity for antiparallel microtubules. This analysis has revealed that the C-terminal domain may interact independently with microtubules while the motor domain is required for maintaining the interaction with the antiparallel microtubules. Surprisingly, deletion of the N-terminal domain resulted in a Subito protein capable of promoting the assembly of bipolar spindles that do not include centrosomes or chromosomes. Bipolar acentrosomal spindle formation during meiosis in oocytes may be driven by the bundling of antiparallel microtubules. Furthermore, these experiments have revealed evidence of a nuclear- or chromosome-based signal that acts at a distance to activate Subito. Instead of the chromosomes directly capturing microtubules, signals released upon nuclear envelope breakdown may activate proteins like Subito, which in turn bundles together microtubules.
TWO events are required to ensure that the pairs of homologous chromosomes properly segregate among two daughter cells at the first meiotic division. First, microtubules must assemble into a bipolar spindle, a process that includes the critical events of establishing bipolarity and the attachment of microtubules to the kinetochores. Second, the homologs must be linked by chiasmata, the result of a crossover formed earlier during prophase. By linking together two homologs, the chiasmata coordinate attachment of homologous kinetochores to microtubules emanating from opposite poles. Since the chiasmata are holding the two homologs together, and the kinetochore microtubules are working to pull them apart, the arrangement remains stable until cohesion is released on the arms of each chromatid, allowing the movement of homolog pairs to the poles.
One mechanism to establish bipolar spindles, and the one commonly observed in mitotically dividing cells, is through centrosome-containing microtubule organizing centers (MTOC). Microtubules growing from the MTOCs at the poles can either attach to a kinetochore (kinetochore microtubules) or interdigitate with microtubules from the opposite pole (interpolar microtubules). Another mechanism, responsible for forming the bipolar spindle in the absence of centrosomes, is observed in the oocytes of many species (Compton 2000; Karsenti and Vernos 2001; Wadsworth and Khodjakov 2004). In these situations, the chromosomes play an important role in the assembly of microtubules into the meiotic spindle. In Drosophila oocytes, for example, nuclear envelope breakdown (NEB) is followed by the accumulation of microtubules around the chromosomes (Theurkauf and Hawley 1992; Matthies et al. 1996). Subsequent bundling and tapering of these microtubules by motor proteins results in a bipolar spindle.
The kinesins are a large family of motor proteins that promote unidirectional movement of a cargo along microtubules and several Drosophila kinesin proteins have been shown to play important roles in spindle assembly (Goshima and Vale 2003). For example, the kinesin-4 or chromokinesin proteins are able to interact with microtubules while attached to chromosomes as their cargo (Mazumdar and Misteli 2005). Another three groups within the kinesin family can bundle and slide parallel or antiparallel microtubules. The first is the kinesin-14 family that includes minus end-directed motors such as NCD in Drosophila. NCD and the minus end-directed motor Dynein have been proposed to bundle and taper microtubules to establish mitotic (Walczak et al. 1998; Goshima et al. 2005) and meiotic (Matthies et al. 1996; Endow and Komma 1997; Skold et al. 2005) spindle poles in the absence of centrosomes. The second is the kinesin-5 family, including Klp61F in Drosophila, which are plus end-directed motors that function to maintain bipolar spindle assembly and elongation at anaphase. The activity of these proteins may antagonize the forces of the kinesin-14 family during spindle assembly (Kwon and Scholey 2004; Tao et al. 2006). The third is the kinesin-6 family that includes Subito and Pavarotti in Drosophila. As shown for human MKLP1, kinesin-6 proteins are thought to be plus end-directed motors that slide antiparallel microtubules (Nislow et al. 1992). Examination of these proteins in human cells (Neef et al. 2003), Caenorhabditis elegans (Raich et al. 1998), and Drosophila (Adams et al. 1998; Cesario et al. 2006) has shown they are usually associated with interpolar microtubules in the middle region of the spindle and are important for cytokinesis. During anaphase, the interpolar microtubules overlap in antiparallel arrays in the spindle midzone, an area that typically accumulates proteins important for cytokinesis (D'avino et al. 2005). Unlike the kinesin-5 and -14 proteins, most studies of kinesin-6 proteins have not implicated them in prometaphase spindle assembly.
In Drosophila, however, the kinesin-6 protein Subito has been shown to have a role in spindle assembly. subito encodes the Drosophila homolog of MKLP2 and has an important role in organizing the meiotic acentrosomal (Jang et al. 2005) and mitotic spindles (Cesario et al. 2006). The Drosophila meiotic spindle develops a prominent bundle of interpolar microtubules during prometaphase, referred to as the metaphase I central spindle, which is a critical part of the acentrosomal spindle assembly pathway (Jang et al. 2005). In subito null mutant oocytes, the central spindle is absent (Jang et al. 2005) and there are an abnormal number of spindle poles and high levels of meiotic nondisjunction (Giunta et al. 2002). Thus, Subito, and by inference the central spindle, is required to organize the acentrosomal spindle during Drosophila female meiosis. Interestingly, the central spindle forms before the microtubules are organized into a bipolar spindle and may function to direct the kinetochore microtubules toward one of the two poles. During mitotic metaphase, Subito may also organize interpolar microtubules but the effect of its absence is much more dramatic in meiosis, possibly because Subito activity is more critical in the absence of centrosomes.
Subito first appears on prometaphase meiotic spindles, suggesting it functions as the microtubules are recruited to the spindle. Just how the microtubules are recruited to surround the chromosomes, however, is poorly understood. The chromosomes could directly interact with microtubules via chromokinesin molecules (Mazumdar and Misteli 2005). Alternatively, the chromosomes could be the source of a signal, such as RanGTP (Clarke 2005), which could activate microtubule assembly factors such as motor proteins. In either case, regulating kinesin proteins like Subito could be particularly important when centrosomes are absent and motor proteins may provide most of the organizing activity. We have characterized the role of the N-, motor, and C-terminal coiled coils domains of Subito and found that regulating Subito activity is a critical component of organizing the acentrosomal spindle. Deregulation of Subito leads to the assembly of microtubules into multiple spindles in the absence of chromosomes or centrosomes. Furthermore, Subito appears to be activated by NEB, suggesting there is a diffusible signal that promotes the bundling of microtubules in oocytes.
MATERIALS AND METHODS
Genetics of sub alleles:
The isolation and genetic analysis of most sub alleles has been described previously (Giunta et al. 2002). These alleles of sub include female sterile mutants sub1, subHM26 (Schupbach and Wieschaus 1989), and sub131 (Giunta et al. 2002); one dominant allele, subDub (Moore et al. 1994); and a fertile hypomorph, sub1794 (Giunta et al. 2002). Two of these mutants, sub1 and sub131, are protein null alleles (Jang et al. 2005).
Generation and initial analysis of transgenic lines:
Full-length and deletion derivatives of subito were amplified by PCR. The clones were verified by sequencing and then the fragments were fused to GFP from pEGFP (Clonetech) at the N terminus using a XhoI or SalI site engineered into the beginning of the subito coding region. The full construct was then subcloned into the pUASP vector (Rorth 1998). In some cases, the Gateway system was used to generate the pUASP clones (T. Murphy, personal communication).
To measure fertility and chromosome segregation during meiosis, females were crossed to y w/BsY males. The nondisjunction frequency was calculated as 2(BS ♀ + B+ ♂) / [B+ ♀ + BS ♂ + 2(BS ♀ + B+ ♂)]. Ovary protein levels were assayed by Western blot. Whole ovaries were dissected from yeasted females in PBS and then ground and boiled in SDS gel loading buffer. Protein from ∼2 to 3 ovaries was loaded per lane. Primary antibodies were rat anti-SUB, rat-anti HA “high affinity” (Roche, clone 3F10), and mouse anti-GFP (Chemicon, clone JL-8), all used at 1:5000; the secondary HRP-conjugated antibodies (Jackson Labs) were used at 1:5000. The secondary was detected using ECL reagents (Amersham, Piscataway, NJ).
Antibodies and immunofluorescent microscopy:
Two methods to isolate oocytes: “mass isolation” and “dissection” were used. In the mass isolation protocol, oocytes were collected by physical disruption. Stage 14 oocytes were collected from 50 to 200 3- to 7-day-old yeast fed females by physical disruption in a common household blender. The details have been described previously (Theurkauf and Hawley 1992), but in short, the oocytes were fixed in modified Robb's media and cacodylate/formaldehyde fixative for 8–10 min and then their outer membranes were removed by rolling the oocytes between the frosted part of a slide and a coverslip. In the dissection protocol, oocytes were hand dissected from 3- to 7-day-old yeast fed females and then fixed using the buffer A protocol (Belmont et al. 1989). In this method, the stage 14 oocytes retain their outer membranes and chorion, blocking the entry of antibodies. The advantage of the dissection protocol, however, is that we were able to isolate a range of oocyte stages. In contrast, the mass isolation procedure resulted in only mature stage 14 oocytes. Thus, for the analysis of P{GAL4∷VP16-nos.UTR}MVD1/P{UASP:GFP-subΔNT} mutant oocytes, the dissection procedure was more effective than the mass isolation procedure at isolating oocytes shortly after NEB.
To depolymerize microtubules, mass isolated stage 14 oocytes were incubated in 50 μl/ml of 10−3 m colchicine solution in Robb's buffer for 1 hr prior to fixation. Control oocytes were incubated for 1 hr in buffer (Robb's) without colchicine.
When examining sub mutant oocytes, heterozygotes for protein null alleles sub1/sub131 were used. Oocytes were stained for DNA with Hoescht and for microtubules with anti-tubulin monoclonal antibody DM1A (at 1:50), in some cases directly conjugated to FITC (Sigma, St. Louis). Heterozygotes were used to eliminate potential genetic background effects. The rat anti-SUB antibody was used at 1:75 combined with either a Cy3 or Cy5 anti-rat secondary antibody absorbed against a range of mammalian serum proteins including mouse and rabbit (Jackson Labs). Additional primary antibodies were the rat anti-HA (Roche, clone 3F10) (1:25), rabbit anti-TACC (1:75) (Gergely et al. 2000), rabbit anti-AurB (1:250), rabbit anti-INCENP (1:250) (Adams et al. 2001), and mouse anti-RCC1 (1:20) (Frasch 1991) with Cy3 conjugated secondary antibodies (Jackson Labs). Images were collected on a Leica TCS SP confocal microscope with a 63×, NA 1.3 lens. Images are shown as maximum projections of complete image stacks followed by merging of individual channels and cropping in Adobe Photoshop.
Western blotting:
Total ovary protein was isolated by dissecting whole ovaries from 10 yeasted females in PBS and then grinding and boiling them in SDS gel loading buffer. Protein from approximately one ovary was loaded per lane. The rat anti-HA (Roche, clone 3F10) or mouse anti-GFP (Clonetech, clone JL-8) primary antibodies were used at 1:5000 and the secondary anti-rat-HRP antibody (Jackson Labs) was used at 1:5000. The secondary was detected using ECL reagents (Amersham).
RESULTS
We have proposed that acentrosomal spindle assembly depends on the bundling of interpolar microtubules. Experiments to test this hypothesis were undertaken with a functional analysis of the Subito protein. By analyzing mutations of Subito, the goal was to identify what determines the specificity for interpolar microtubules and what controls when and how Subito interacts with the meiotic spindle.
Subito localization in oocytes is dependent on microtubules:
As oocytes enter stage 14, the nuclear envelope breaks down and microtubules immediately begin to assemble around the chromosomes (Matthies et al. 1996). There is no congression of bivalents since the chromosomes begin prometaphase in a single condensed mass or karyosome. The meiotic spindle assembles around this structure. Subito colocalizes with meiotic spindle microtubules from the earliest stages of spindle assembly, although always immediately adjacent to the karyosome (Jang et al. 2005) (Figure 1).
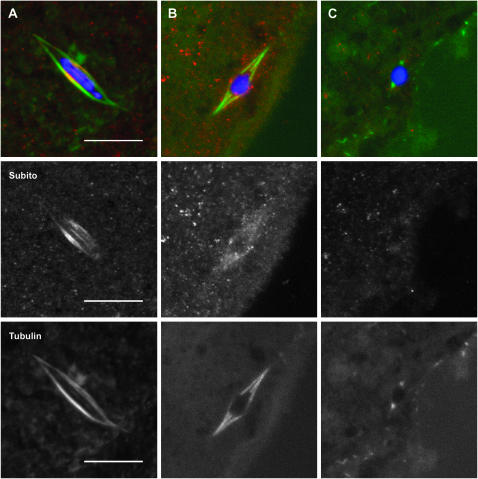
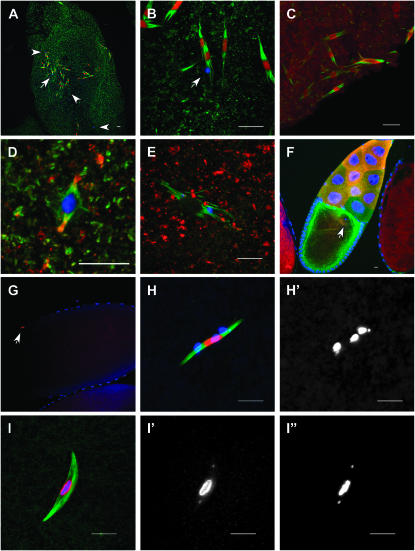
Figure 1.—
Subito localization to the central spindle is microtubule dependent. (A) Wild-type stage 14 oocyte treated in buffer without colchicine. (B and C) Stage 14 oocytes treated with colchicine for 1 hr. DNA is in blue, Subito in red, and tubulin in green. The Subito and Tubulin channels are shown below the merged images. Note that the brightest tubulin fluorescence is present in the central spindle of the control spindle, but this region shows the least intense fluorescence when colchicine treated. Subito is either absent or uniformly localized following colchicine treatment. The high background in B is due to optimizing on the low Subito signal. Bar, 10 μm.
Since Subito colocalizes with microtubules but only adjacent to the chromosomes, we tested their relative importance by treating stage 14 oocytes with colchicine to depolymerize the microtubules. All control oocytes (7/7) incubated in buffer without colchicine had normal spindle structure, in which the brightest tubulin staining was in the central spindle region (Figure 1A). The bright tubulin staining was most likely due to the overlap of interpolar microtubules, which also colocalize with Subito staining. Colchicine treatment of stage 14 oocytes most frequently resulted in partial loss of the microtubules (Figure 1, B and C). While colchicine treatment will completely depolymerize spindle microtubules in larval brains, the partial loss of the microtubules in colchicine-treated live oocytes could have been due to limited permeability since they still possessed their vitelline membrane and chorion. Despite partial depolymerization, the central spindle went from the brightest part of the spindle to the dimmest in all the examined oocytes (12/12), suggesting that interpolar microtubules were more sensitive to colchicine than the kinetochore microtubules. In addition, and regardless of whether there was a bipolar spindle, none of the colchicine-treated oocytes had Subito staining visible in the usual pattern. In some colchicine-treated oocytes, faint Subito staining was localized along the length of the spindle but it was never concentrated in the center as in untreated oocytes. Thus, Subito localization depends on the microtubules and may localize to kinetochore microtubules when interpolar microtubules are absent. What restricts Subito to interpolar microtubules and adjacent to the chromosomes is the subject of the remaining experiments.
Analysis of Subito function using epitope-tagged transgenes:
To investigate what controls Subito localization, we examined the function of mutated derivatives of sub (Figure 2). Subito contains a central motor domain flanked by two poorly conserved domains. Transgenes were made by cloning wild-type sub sequence or variants containing a mutation in one of the domains into the pUASP vector, which contains multiple copies of the UAS sequence in the promoter. This permitted expression in Drosophila that was controlled with a second transgene containing GAL4 fused to a Drosophila promoter (Rorth 1998). In all the experiments discussed below, the UASP:sub transgenes were expressed using the P{GAL4∷VP16-nos.UTR}MVD1 driver, which has GAL4 fused to the nanos promoter and induces the expression of UAS containing transgenes in the female germline (Rorth 1998). For each transgene, at least two and usually more insertion lines were examined in case expression levels from different insertion sites were different. In most cases, with the exceptions noted below, differences in expression levels as assayed by Western blot were minimal and not the explanation for mutant phenotypes (supplemental Figure S1 at http://www.genetics.org/supplemental/).
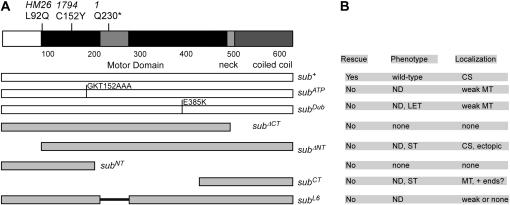
Figure 2.—
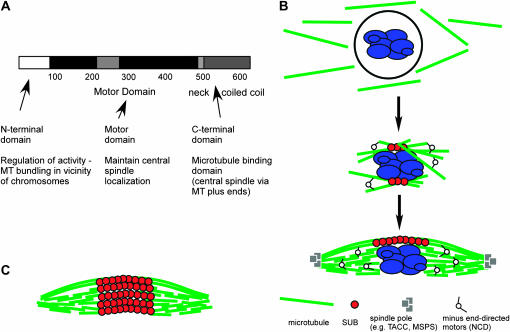
Structure of wild-type and mutant Subito transgenes and summary of phenotypes. (A) The conserved motor domain is shown in black while the less conserved regions are shown in lighter shading. This includes an insertion into the motor domain found only in kinesin-6 family members. (B) Effects of the different transgenes are summarized. ND, dominant nondisjunction; LET, recessive lethal; ST, dominant sterility; CS, central spindle; MT, all microtubules; ectopic, extra spindles.
The phenotype of wild-type and mutant sub transgenes was characterized using genetic and cytological assays. Genetics assays were performed to examine two functions of Subito in meiotic chromosome segregation and early embryonic development. By expressing the transgenes in a genetic background with a null allele (sub1 or sub131, herein referred to as subnull) and scoring the fertility of the females, we were able to determine if a mutation affected the Subito embryonic function. Females homozygous for sub null alleles are sterile due to a maternal requirement for Subito early in embryogenesis (Schupbach and Wieschaus 1989; Giunta et al. 2002). By expressing the transgenes in a genetic background with a sub hypomorphic allele (sub1794/subnull) and measuring the frequency of X chromosome nondisjunction (see materials and methods), we were able to determine if a mutation affected the Subito meiotic chromosome segregation function. Sub1794 is a hypomorph that is fertile but exhibits a high frequency of meiotic nondisjunction when homozygous or heterozygous with a null allele (Table 1). Similarly, by expressing a transgene in wild-type females and measuring fertility and the frequency of X chromosome nondisjunction, dominant effects on chromosome segregation could be detected. Transgenes expressing the full-length Subito protein (P{UASP:sub+}) fused to GFP or an HA tag almost completely rescued the meiotic (Table 1) and sterile (Tables 2 and 3) sub mutant phenotypes.
TABLE 1.
Rescue of meiotic nondisjunction phenotype by sub transgenes
| Transgenea | sub genotype | Regular progeny | Nondisjunction progeny | Female parents | Progeny/female parent | Nondisjunction (%) |
|---|---|---|---|---|---|---|
| None | sub1794/subnull | 1888 | 391 | 146 | 15.6 | 29.3 |
| P{UASP:GFP-sub+}35 | sub1794/subnull | 2161 | 26 | 30 | 72.9 | 2.3 |
| +/+ | 1074 | 16 | 20 | 54.5 | 3.0 | |
| P{UASP:GFP-subCT}43 | +/+ | 243 | 13 | 38 | 6.7 | 9.7 |
| subnull/+ | 209 | 13 | 14 | 15.8 | 11.1 | |
| sub1794/subnull | 0 | 0 | 30 | 0 | — | |
| P{UASP:GFP-subΔNT}31 | +/+ | 0 | 0 | 40 | 0 | — |
| subnull/+ | 60 | 1 | 40 | 1.5 | 3.2 | |
| sub1794/subnull | 180 | 3 | 56 | 3.3 | 3.2 |
For each transgene, data for one example insertion are shown. At least one additional insertion for each transgene gave similar results.
Each transgene was expressed by crossing to P{GAL4∷VP16-nos.UTR}MVD1.
TABLE 2.
Rescue of sterility in cn sub1 bw/sub131 bw mutants by sub transgenes
| Transgenea | Regular progeny | Nondisjunction progeny | Female parents | Progeny/female parent | Nondisjunction (%) |
|---|---|---|---|---|---|
| P{UASP:GFP-sub+}35 | 1373 | 18 | 36 | 77.3 | 2.5 |
| P{UASP:GFP-subΔNT}31 | 0 | 0 | 20 | 0 |
Control females of the genotype cn sub1 bw/sub131 bw; P{GAL4∷VP16-nos.UTR}MVD1/+ or cn sub1 bw/sub131 bw; +/P{UASP:GFP-sub+}35 were sterile. For each transgene, data for one example insertion are shown. At least one additional insertion for each transgene gave similar results.
Each transgene was expressed by crossing to P{GAL4∷VP16-nos.UTR}MVD1.
TABLE 3.
Dominant effects of sub motor domain mutants
| Transgenea | sub genotype | Regular progeny | Nondisjunction progeny | Nondisjunction (%) |
|---|---|---|---|---|
| P{UASP:sub+HA}31 | +/+ | 1254 | 1 | 0.2 |
| sub1/sub131 | 2518 | 6 | 0.5 | |
| sub1/+ | 1932 | 1 | 0.1 | |
| P{UASP:subATP}4 | +/+ | 2483 | 173 | 12.2 |
| sub1/+ | 608 | 166 | 32.8 | |
| P{UASP:subL6}10 | +/+ | 4550 | 0 | |
| sub1/+ | 2189 | 100 | 8.4 | |
| P{UASP:GFP-subNT}28 | sub1/+ | 1900 | 8 | 0.8 |
Control females of the genotype cn sub1 bw/sub131 bw; P{GAL4∷VP16-nos.UTR}MVD1/+ or cn sub1 bw/sub131 bw; +/P{UASP:sub+HA}31 were sterile.
Each transgene was expressed by crossing to P{GAL4∷VP16-nos.UTR}MVD1.
Cytological assays were performed on the same wild-type and mutant sub transgenes to examine their effects on spindle assembly and Subito localization. Using tubulin staining, we could determine if a mutant affected spindle organization. For example, sub mutant oocytes typically exhibit monopolar, tripolar, and frayed spindles (Giunta et al. 2002; Jang et al. 2005). Protein expressed from the full-length Subito transgene (P{UASP:GFP-sub+}) fused to GFP or an HA tag localized to the same region of the meiotic metaphase I spindle in stage 14 oocytes as the endogenous Subito protein (e.g. Figure 3A) and rescued the spindle organization defects of sub mutants (see below).
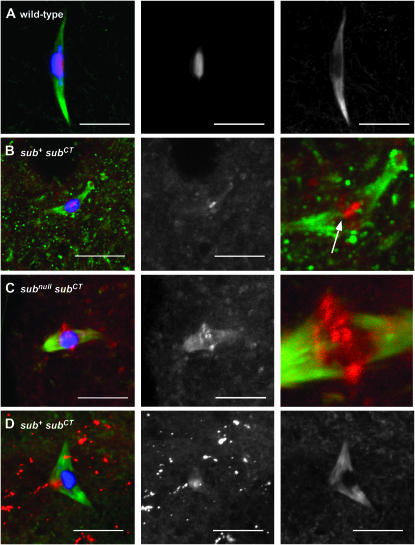
Figure 3.—
Localization of full-length GFP-tagged Subito and C-terminal domain proteins in stage 14 oocytes. In all the experiments discussed below, the transgenes were expressed using the P{GAL4∷VP16-nos.UTR}MVD1 driver. DNA is in blue, Subito or GFP-Subito in red, and tubulin in green. The second column shows the Subito signal from GFP (A–C) or antibody staining (D). Tubulin, along with Subito in some cases, is shown in the last column. (A) Full-length Subito fused to GFP (P{UASP:GFP-sub+}). (B) The C-terminal domain of Subito fused to GFP (P{UASP:GFP-subCT}). These females also expressed wild-type Subito from the endogenous locus. In the last column is a magnification with the DNA staining removed to show that the central focus of GFP-SubitoCT staining (arrow) is associated with thin microtubule fibers. (C) The C-terminal domain of Subito fused to GFP and expressed in the absence of wild-type Subito (sub1/sub131; P{UASP:GFP-subCT}). Also shown is a magnification of the region near the karyosome with DNA staining removed to show GFP-SubitoCT staining associated with microtubule fibers. In B and C, the weak GFP staining was also observed in experiments without staining for tubulin, showing that the signal was not due to bleed through (data not shown). (D) The C-terminal domain of Subito fused to GFP and expressed in the presence of wild-type Subito (P{UASP:GFP-subCT}) and stained with an antibody that recognizes both the wild-type and SubitoCT proteins. As in Figure 1B, the high background is due to optimization for the low Subito signal. Bars, 10 μm.
The C-terminal domain colocalizes with spindle microtubules:
The function of the C-terminal domain of Subito was investigated with construct P{UASP:GFP-subCT}, which contained only the C-terminal domain of sub fused to GFP (Figure 2). Immunofluorescence of P{UASP:GFP-subCT} stage 14 oocytes revealed that the C-terminal fragment of Subito colocalized with the microtubules (Figure 3). Therefore, the C-terminal domain appears to have microtubule-binding activity independent of the motor domain. However, the intensity of GFP-SubitoCT staining varied depending on the presence or absence of wild-type protein. In the presence of wild-type Subito, the staining that colocalized with tubulin was weak (Figure 3B). In addition, there was also a small dot of stronger staining that colocalized with the chromosomes. The nature of this structure is not known but it did appear to associate with microtubule fibers. In the absence of wild-type Subito (e.g., subnull females), GFP-SubitoCT staining with the microtubules was more intense (Figure 3C), suggesting the substrate that GFP-SubitoCT binds to may be more abundant in subnull oocytes. SubitoCT and the wild-type protein may compete for binding on the spindle or a particular type of binding site may be more abundant in sub mutants, such as free microtubule plus ends (Jang et al. 2005). Interestingly, GFP-SubitoCT was often enriched on the microtubules closest to the chromosomes, indicating that the C-terminal domain could specify a preference for microtubule plus ends. The C-terminal domain may also be important for protein stability since protein expression from a construct lacking this domain, P{UASP:GFP-subΔCT}, was not detected by either Western blot or immunofluorescence (supplemental Figure S1 at http://www.genetics.org/supplemental/).
Given the absence of the motor domain, it was not surprising that UASP:GFP-subCT did not rescue sub mutant phenotypes (Table 1). More interestingly, expression of the GFP-SubitoCT fragment had dominant effects on chromosome segregation and fertility. In otherwise wild-type females, expression of GFP-SubitoCT caused high levels of X chromosome nondisjunction (Table 1). GFP-SubitoCT also affected fertility, causing reduced progeny numbers in sub1794/+ females and sterility in sub1/sub174 females. These results suggest that the C-terminal fragment can interfere with the activity of the wild-type protein.
The dominant effects of SubitoCT on chromosome segregation were mirrored by the effects of GFP-SubitoCT on spindle morphology. When GFP-SubitoCT was expressed in a wild-type background, 8/10 oocytes had abnormal microtubule organization such as tripolar, monopolar, or frayed spindles or spindles lacking interpolar microtubules, effects similar to, although milder than, those found in the null mutants. To determine if the GFP-SubitoCT dominant phenotypes resulted from effects on the wild-type protein, the oocytes expressing GFP-SubitoCT were stained for the wild-type protein. Localization of wild-type Subito to the central spindle was reduced or not detected in 5 of the 10 spindles (Figure 3D). In contrast, all 10 control oocytes (expressing wild-type GFP-Subito) localization of the endogenous Subito protein was not affected. Thus, GFP-SubitoCT may interfere with the localization and function of wild-type Subito, resulting in loss-of-function phenotypes.
In summary, the C-terminal domain of Subito localizes with spindle microtubules. It may also be able to compete with the wild-type protein for binding sites, resulting in the formation of abnormal spindles and elevated rates of chromosome segregation errors.
The Subito motor domain is required for localization to the central spindle:
To investigate the importance of the motor activity for Subito function, we characterized four point mutations and one internal deletion in the motor domain of subito (sub1794, subhm26, SubDub, subATP, and subL6, Figure 2). In sub1794 and subhm26 mutants, the Subito localization pattern was similar to wild type, although Subito protein levels were reduced in subhm26 mutants. This was confirmed on a Western blot (data not shown), suggesting the Subitohm26 protein could localize but was unstable.
In subDub, an invariant amino acid of the motor domain is changed (Figure 2) (Giunta et al. 2002), causing a dominant meiotic nondisjunction phenotype in both males and females (Moore et al. 1994) and defective spindle assembly (Giunta et al. 2002). SubDub homozygotes are lethal but subDub/subnull females are viable, allowing for the analysis of meiosis in females where the only source of Subito protein was from the Dub allele. Using the polyclonal antibody, the majority of subDub/subnull stage 14 oocytes had abnormal Subito staining. In 10/18 oocytes, the SubitoDub protein was either weakly distributed along the spindle or concentrated toward the poles. In the remaining 8/18 oocytes, no Subito staining was detected (Figure 4B) even though the protein was readily detected on a Western blot (data not shown). Most (13/18) of these oocytes had abnormal spindle morphology. These results indicate that the subDub mutation affected the localization of the protein to interpolar microtubules on the spindle.
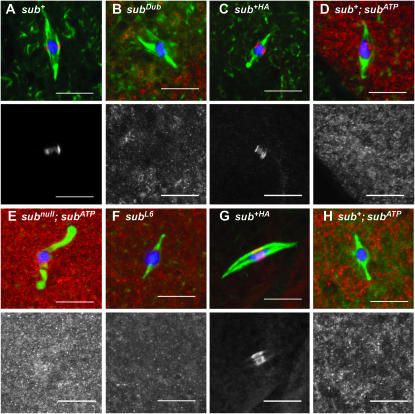
Figure 4.—
Localization of Subito in stage 14 oocytes of motor domain mutants. In all the experiments discussed below, the transgenes were expressed using the P{GAL4∷VP16-nos.UTR}MVD1 driver. DNA is in blue, Subito in red, and tubulin in green. The Subito channel is shown below each color image. (A) Subito detected using an anti-Subito antibody in a wild-type oocyte. (B) Subito detected using an anti-Subito antibody in a subDub/sub1 oocyte. (C) Subito detected using an antibody to the HA tag in an oocyte expressing wild-type sub fused to an HA epitope tag (P{UASP:sub+HA}) or (D) a similar HA-fusion protein with a mutation in the ATP binding domain (P{UASP:subATP}) in a wild-type background or (E) subATP in a subnull mutant background. (F) Subito detected using an anti-HA antibody in an oocyte expressing a HA-fusion protein lacking 60 amino acids present in the motor domain of all kinesin-6 family members (P{UASP:subL6}) in a wild-type background. (G) Subito detected using an anti-Subito antibody in sub+; P{UASP: sub+HA} or (H) sub+; P{UASP:subATP} oocytes. Bar, 10 μm.
Since the biochemical defect of subDub has not been characterized, we generated a mutant in the ATP-binding domain (Figure 2) with the objective of eliminating motor activity but not microtubule binding (e.g., Zhu and Jiang 2005). This mutant, subATP, did not rescue the sterility of sub null mutants and had similar dominant phenotypes as subDub mutants. For example, expression of the mutant protein in a wild-type background resulted in chromosome segregation errors, suggesting that the mutant had a dominant negative effect (Table 3). Consistent with these genetic results, analysis of the meiotic spindles in subATP females revealed abnormal albeit mild defects in microtubule organization. A total of 16/38 spindles were judged to be abnormal because they were frayed, lacked interpolar microtubules, were unusually curved, or contained asymmetries such as large knob structures. In contrast, 7/7 control oocytes (expressing the wild-type P{UASP-sub+HA} transgene) had normal bipolar spindles. Similar to subDub, microtubule-associated staining of the mutant SubitoATP protein was reduced; only faint staining coincided with the microtubules of the meiotic spindle (Figure 4, D and E). A Western blot indicated that the SubitoATP protein was expressed at normal levels (supplemental Figure S1 at http://www.genetics.org/supplemental/), suggesting that ATP hydrolysis is essential for protein localization.
The effect on interpolar spindles in these oocytes suggests that the SubitoATP protein may have disrupted the function of the wild-type protein. To test this, we stained subATP; sub+ oocytes with our polyclonal antibody for the endogenous Subito protein and found it was reduced by expression of SubitoATP (Figure 4H). Among the 38 oocytes characterized above for spindle morphology, 22 lacked endogenous Subito staining and in many others Subito staining was weak. These results indicated that SubitoATP negatively impacted on the localization of wild-type protein, which was probably a contributing factor in the dominant effects on chromosome segregation.
Motor function was also investigated with the analysis of a mutation (subL6), which removed 60 amino acids present in the motor domain of all kinesin-6 family members but no other kinesin-like proteins (Figure 2). Even though none of the motor domain sequences conserved in other kinesins were deleted in the subL6 mutation, there was little evidence of microtubule binding. Spindle staining by immunofluorescence was absent (Figure 4F) even though the protein was easily detected by Western blotting (supplemental Figure S1 at http://www.genetics.org/supplemental/). Therefore, this portion of the motor domain appears to be essential for localization to the spindle. The mutant transgenes did not rescue the sterility of sub null alleles and when expressed in a wild-type background did not cause meiotic nondisjunction, suggesting the mutant protein was nonfunctional. However, expressing subL6 in a background where wild-type subito dosage was reduced (e.g., subnull/+) resulted in increased meiotic nondisjunction (Table 3), suggesting that Subito was present in limiting amounts in the oocyte. Similarly, the subATP mutant phenotype was more severe when sub dosage was reduced.
In summary, three mutants affecting different parts of the motor domain had qualitatively similar effects. They caused dominant negative phenotypes, including chromosome segregation errors, spindle assembly defects, and reduced localization of the wild-type protein. The mutant proteins also failed to localize correctly on the metaphase spindle, but the dominant negative phenotypes suggested that the mutant motor domain mutant proteins could interact with the spindle in ways that interrupt localization of the wild-type protein.
The N-terminal domain regulates Subito localization:
Two constructs were made to investigate the function of the N-terminal domain. The first, P{UASP:GFP-subNT}, contained the N-terminal domain without the motor. When crossed to P{GAL4∷VP16-nos.UTR}MVD1, protein was not detected in stage 14 oocytes by immunofluorescence. Thus, the N-terminal domain lacks sequences that direct localization. In addition, expression of this fragment did not have effects on meiotic chromosome segregation or fertility (Table 3). Since the protein levels were reduced in this mutant (supplemental Figure S1 at http://www.genetics.org/supplemental/), however, it is possible that GFP-subNT was not expressed at a high enough level to act as a dominant negative.
The second construct, P{UASP:GFP-subΔNT}, was a deletion of the N-terminal domain. Immunofluorescence analysis in oocytes demonstrated that this fragment of Subito had a dramatic effect on spindle formation. Stage 14 oocytes expressing P{UASP:GFP-subΔNT} contained a large number of bundled microtubules that appeared like bipolar spindles (Figure 5A). On the basis of Western blot analysis, the presence of ectopic spindles cannot be attributed to higher expression levels. This phenotype was identical in the presence or absence of wild-type Subito protein and was observed in all oocytes examined but never in control oocytes. Furthermore, most of these “ectopic” spindles were not associated with chromosomes (Figure 5B). Indeed, the karyosome had usually prematurely split into at least two masses of chromosomes that were scattered in the oocyte cytoplasm. Thus, microtubules assembled into bipolar spindles without chromosomes and centrosomes.
Figure 5.—
Subito lacking the N-terminal domain promotes formation of ectopic spindles. In all the experiments discussed below, the transgenes were expressed using the P{GAL4∷VP16-nos.UTR}MVD1 driver. DNA is blue; Subito-GFP, TACC (D and E), or Aurora B (C) is red, and tubulin is green. (A) Stage 14 oocyte expressing Subito with a deletion of the N-terminal domain fused to GFP (P{UASP:GFP-subΔNT}). The locations of chromosome masses are shown by the arrow and arrowheads. While these images are taken from oocytes also expressing wild-type Subito protein, similar effects were observed in oocytes lacking wild-type Subito protein. (B) The same oocyte as shown in A with the region around the arrow magnified. (C) GFP-subΔNT stage 14 oocyte stained for Aurora B (red). (D) In wild-type metaphase TACC (red) is visible at the poles. (E) In GFP-subΔNT ectopic spindles TACC (red) is absent. (F) In GFP-subΔNT stage 10 oocytes, there are no ectopic spindles. The oocyte karyosome is shown by an arrow. The cortical green staining is from microtubule growth that is nucleated from many organizing centers along the cortex of the Drosophila oocyte prior to NEB as part of the axis specification during oogenesis (Theurkauf et al. 1992; Cha et al. 2002; Steinhauer and Kalderon 2006). (G) Early GFP-subΔNT stage 14 oocyte with only one spindle, as indicated by a single patch of Subito staining (arrow). In the method used to prepare this oocyte, tubulin staining was not possible (see materials and methods). (H) A different GFP-subΔNT early stage 14 oocyte than in G, showing the premature separation of the karyosome. This was the only spindle in the oocyte. (H′) DNA channel, showing the separated small fourth chromosomes as well as the three remaining masses that are probably bivalents held together by chiasma. (I) Wild-type stage 14 oocyte stained for RCC1 (red). Separate channels for RCC1 (I′) and DNA (I″) show that even the small fourth chromosomes stain with RCC1. Bars, 10 μm.
These results suggest that the N-terminal domain regulates Subito activity and as a consequence spindle formation. The N-terminal domain is required to ensure that microtubules are only assembled around the chromosomes. The SubitoΔNT protein may promote the unregulated bundling of microtubules. In support of this conclusion, we observed the GFP-SubitoΔNT protein in the central region of each ectopic spindle, even in the absence of wild-type protein (Figure 5B). To determine what other proteins were recruited to these spindles, we stained for the passenger proteins Incenp and Aurora B, which normally localize to the central region of wild-type spindles (Jang et al. 2005). Like Subito, Incenp and Aurora B were also found at the center of the ectopic spindles, suggesting the complex of proteins that forms in the meiotic central spindle of wild-type oocytes was also forming on these ectopic spindles (Figure 5C).
Another feature of meiotic spindles is the localization of TACC and MSPS to the poles even though centrosomes are absent (Cullen and Ohkura 2001). To examine the structure of the poles in the ectopic spindles assembled as a result of GFP-SubitoΔNT activity, we stained P{GAL4∷VP16-nos.UTR}MVD1; P{UASP:GFP-subΔNT} oocytes with an antibody to TACC, but no staining was detectable at the poles (Figure 5, D and E). The absence of TACC (and presumably other proteins like MSPS) at the poles may be the reason the spindles assembled as a result of GFP-SubitoΔNT activity were often splayed at the poles. From a sample of 32 spindles (i.e., 64 poles), 70% were splayed while 30% were finely tapered similar to wild type. The two poles of a spindle were often different, with 34% having one splayed and one tapered pole.
To examine the time course of ectopic spindle formation in P{UASP:GFP-subΔNT} oocytes, we isolated oocytes from all stages of development (see materials and methods). A key event is NEB, which occurs in mature oocytes at the beginning of stage 14. In stage 13 or earlier stage oocytes, which is prior to NEB, ectopic spindles were not observed (Figure 5F). Although the mutant GFP-SubitoΔNT protein and microtubules were abundant in the oocyte cytoplasm prior to stage 14, they apparently did not interact. After NEB, spindle assembly in the earliest stage 14 oocytes was restricted to the vicinity around the chromosomes (Figure 5, G and H). In addition, these early stage 14 oocytes often had multiple chromosome masses, suggesting that spindle assembly in the presence of GFP-SubitoΔNT caused the karyosome to split prematurely, which is not usually observed until anaphase I. This would explain the observation that the chromosomes appeared scattered in the oocyte cytoplasm in older stage 14 oocytes. The simplest interpretation of these data is that the activity of the GFP-SubitoΔNT protein is dependent on NEB. Shortly after NEB, GFP-SubitoΔNT protein and microtubules begin to assemble into spindles in the vicinity of the chromosomes. The ectopic spindles continue to form such that in older stage 14 oocytes, they become numerous and less restricted in position. They also were observed in embryos, which in wild type is after the time when anaphase I and meiosis II occur (supplemental Figure S2 at http://www.genetics.org/supplemental/).
As described in the discussion, the chromosome-associated protein RCC1 could be one of the factors that promotes spindle assembly following NEB. In the presence of RCC1, Ran is converted into its active form (GTP bound), which can then promote microtubule assembly. Drosophila RCC1 has been reported to be present in the oocyte nucleus (Frasch 1991) and we have confirmed it is chromosome associated after NEB (Figure 5I).
GFP-subΔNT did not rescue the sterile phenotype of subnull females (Table 2). Indeed, GFP-subΔNT had a dominant effect on fertility, the severity of which was dependent on the dosage of wild-type sub gene product. Females expressing GFP-SubitoΔNT in a subnull/+ or a +/+ background had lower fertility than in a sub1794/subnull background. In contrast, genetic assays measured a surprisingly low frequency of meiotic nondisjunction when GFP-SubitoΔNT was expressed in a sub1794/subnull mutant (Table 1). It is possible, however, that this experiment scored a selected set of oocytes since expression of GFP-subΔNT had a dominant effect on female fertility.
While the motor and C-terminal domains are involved in the specificity of Subito localization, the N-terminal domain of Subito appears to regulate its activity in bundling microtubules. Spindle assembly in the presence of this mutant form of Subito is not limited to a single structure adjacent to the karyosome. The activity of this protein also appears to cause the karyosome to prematurely separate.
DISCUSSION
Subito is a kinesin motor protein that contributes to acentrosomal meiotic spindle formation (Giunta et al. 2002), possibly by stabilizing the overlap of antiparallel microtubules located in the central spindle during meiotic metaphase (Jang et al. 2005). The object of this study was to investigate the characteristics of Subito that facilitate spindle formation (Figure 6). The results have stimulated a model for acentrosomal spindle assembly that emphasizes the bundling of antiparallel microtubules without direct contacts with the chromosomes.
Figure 6.—
Model for function of Subito in spindle assembly. (A) Schematic of Subito and summary of the functions attributed to each domain. (B) Prior to NEB, Subito does not interact with the microtubules. After NEB, Subito associates with microtubules early in prometaphase. Initially, the majority of microtubules may only interact laterally with the chromosomes (Skold et al. 2005). There may also be kinetochore microtubules forming concurrently (Jang et al. 2005) but the relative timing is not known. In a model for acentrosomal spindle assembly that involves the capture and bundling of microtubules, stabilized plus and minus ends would be expected throughout the spindle, not just near the chromosomes (Jang et al. 2005). Indeed, there is experimental evidence for both microtubule plus and minus ends localized throughout meiotic spindles (Elliott et al. 2005; Burbank et al. 2006). (C) A model for the structure of the spindles when GFP-SubitoΔNT was expressed.
Localization of Subito depends on the C-terminal domain:
When a fragment containing only the C-terminal domain of Subito was expressed, the protein localized to the spindle microtubules. It is also likely that that there is competition between the C-terminal domain and full-length Subito for binding sites. This is based on the observation that when the C-terminal domain was expressed in a wild-type background, there was less wild-type protein localized to the central spindle at metaphase I and an increased incidence of chromosome segregation errors. Similarly, the C-terminal domain of MKLP2 has been shown to have microtubule binding activity in vitro (Echard et al. 1998) and in vivo (Gruneberg et al. 2004). These results indicate that Subito, and other MKLP2 paralogs, have two microtubule-binding domains, one each in the C-terminal and motor domains. This feature of the MKLP2 proteins may enable them to form cross-bridges between antiparallel microtubules (Nislow et al. 1992). Specificity may also reside in the protein–protein interactions involving Subito. Similar to MKLP2, the C-terminal domain of Subito may interact with the Passenger proteins and CDC14 (Gruneberg et al. 2004). Indeed, the passenger proteins and Subito or MKLP2 may be obligate partners during meiosis (Jang et al. 2005) and mitosis (Cesario et al. 2006; Neef et al. 2006).
Subito motor domain is required to maintain the interaction with microtubules:
We characterized two mutations that affect conserved amino acids in the motor domain and a third that affects a motor domain sequence specific to kinesin-6 proteins. All three mutants exhibited dominant nondisjunction and weak spindle staining, suggesting they had similar defects in motor activity. The subDub mutation changes an E to K at position 385. Although this mutation is outside of the microtubule-binding region (Vale and Fletterick 1997), it is the last residue in a group of seven amino acids that are invariant in all kinesin-like proteins. This mutant has a dominant nondisjunction phenotype (Moore et al. 1994) and the protein fails to accumulate in the central spindle. The failure to localize to microtubules is surprising because similar mutants (E → A) in minus end-directed motors such as Kar3 or NCD bind strongly to microtubules but lack a microtubule-stimulated ATPase activity (Yun et al. 2001). Thus, the SubitoDub protein is predicted to bind microtubules but have an inactive motor. A similar array of phenotypes was observed when we generated a mutation that changes the three invariant amino acids GKT in the ATP-binding domain to AAA (subATP). This change has been made in other kinesins (e.g., Zhu and Jiang 2005) and similar changes of the GKT sequence have been made in Pavarotti (EKT) (Minestrini et al. 2002) and the kinesin-5 homolog Eg5 (GKN, GKI) (Blangy et al. 1998) or kinesin heavy chain (GKN) (Nakata and Hirokawa 1995). Most of these mutants exhibited “rigor” binding phenotypes associated with excessive binding of microtubules in vivo. In contrast, the SubitoATP protein failed to localize strongly to meiotic spindles.
Despite the weak localization of the motor domain mutants, several observations indicate these motor domain mutant proteins interact with the spindle microtubules. First, these mutants have dominant effects on meiotic chromosome segregation and spindle organization. Second, these mutants cause reductions in the localization of wild-type protein to the spindle. Indeed, interfering with the localization of wild-type Subito protein could be the cause of the dominant nondisjunction phenotype. Third, at least one of the motor defective proteins (SubitoATP) localizes strongly to metaphase microtubules in mitotic cells, although not to the central spindle like wild-type protein (J. Cesario and K. McKim, unpublished data). These observations suggest that in the presence of motor domain defective proteins, wild-type Subito engages in interactions that lead to its removal from the spindle.
Motor defective Subito protein may be able to initially associate with the microtubules but then be rapidly displaced toward the poles. This would explain the observation that motor domain mutant proteins fail to localize on the spindle despite containing an intact C-terminal domain that can independently interact with the spindle (compare Figure 3C and Figure 4D). Such a “polar wind” has been implicated in previous studies of Drosophila oocytes (Carpenter 1991; Cullen and Ohkura 2001). It is also possible that the motor-inactive Subito proteins could be dislodged from the spindle by another mechanism. Whatever the mechanism by which the motor domain mutant proteins fail to remain on the spindle, these results suggest that the motor domain is required to retain Subito on the spindle in oocytes.
The N-terminal domain is one of at least two factors that regulate Subito activity:
We have identified two factors that regulate Subito, by characterizing stage 14 oocytes expressing a Subito mutant lacking the N-terminal domain (SubitoΔNT). The first regulator of Subito is shown by the observation that the subΔNT mutant formed a large number of ectopic spindles, indicating there is a mechanism to limit where Subito interacts with microtubules. The second regulator of Subito is shown by the observation that the unregulated microtubule bundling activity in subΔNT mutants was dependent on NEB. This is a different result from overexpressing the kinesin-6 member Pavarotti, which was observed to have effects on oogenesis prior to NEB (Minestrini et al. 2002). Possibly, NEB releases a diffusible factor into the cytoplasm that activates Subito microtubule binding and bundling.
Aside from being numerous, the most striking aspect of the ectopic spindles of subΔNT mutants was that they were not built around chromosomes (Figure 6C). We suggest that, through a still-unknown mechanism, the N-terminal domain regulates Subito activity to ensure that microtubules are bundled only in the direct vicinity of the chromosomes. The N-terminal domain could regulate Subito activity in a spatial manner. For example, this domain could promote interactions with a membranous sheath that has been proposed to surround the developing spindle (Kramer and Hawley 2003). Interestingly, the initial studies of the Subito homolog MKLP2 demonstrated an interaction with Rab6, a Golgi-associated Rab protein, although through the C-terminal domain of MKLP2 (Echard et al. 1998). Another possible mechanism is that the N-terminal domain may respond to a diffusible substance from the karyosome (see below).
Rather than regulate when or where the motor is active, the N-terminal domain could affect the biochemical activity of the motor. For example, unregulated plus end-directed motor activity could lead to lengthening of the spindle through the sliding of antiparallel microtubules, causing the karyosome to be pulled apart and leaving the chromosomes scattered in the oocyte cytoplasm. The scattered chromosomes could go through repeated cycles of stimulating microtubule assembly followed by detachment from the spindle to generate the ectopic spindles observed in subΔNT oocytes. More studies, including understanding the details of karyosome structure and the biochemical properties of Subito and the SubitoΔNT mutant, are needed to distinguish these possibilities.
Summary—the role of Subito in microtubule recruitment and assembly:
We have suggested that the antiparallel overlaps of microtubules in the central spindle play an important role early in spindle assembly (Jang et al. 2005) (Figure 6). Our results are also consistent with previous studies suggesting that interpolar microtubules are more sensitive to destabilizing agents like temperature and colchicine than kinetochore microtubules (Brinkley and Cartwright 1975; Salmon and Begg 1980). Subito is critical for the central spindle since it is required for the interpolar microtubules. Like other members of the kinesin-6 family (Nislow et al. 1992), Subito probably cross-links antiparallel microtubules. Subito has two microtubule binding domains, which may cooperate to facilitate interactions with antiparallel microtubules. In addition, motor activity may play a role in the localization of Subito. However, previous studies have also suggested that spindle assembly in Drosophila oocytes involves the recruitment of microtubules by the chromosomes (Theurkauf and Hawley 1992) and subsequent bundling of parallel microtubules by motor proteins such as NCD (Matthies et al. 1996; Skold et al. 2005).
Spindles appear in subΔNT mutant oocytes, however, that do not have direct contacts to the chromosomes. Therefore, conditions exist in the Drosophila oocyte cytoplasm in which recruitment and assembly of microtubules into a spindle may occur without direct contacts with the chromosomes. The concept of a cytoplasmic state permissive to spindle assembly has been proposed in Xenopus oocytes to explain how the injection of DNA can stimulate spindle assembly but only in cytoplasm from M phase eggs. Normally, however, chromosomes are needed to stimulate the process, leading to the idea that there is an “organizational field” around the chromosomes (Karsenti and Vernos 2001). This two-component model of acentrosomal spindle assembly is consistent with our observations. Alternatively, we cannot rule out that one signal in high concentration near the chromosomes is responsible for generating both the permissive cytoplasmic state for spindle assembly and the organizational field around the chromosomes. However, the results from the subΔNT mutant, which interacts with microtubules but is not restricted to the chromosomes, suggest activation is separable from restriction around the chromosomes.
Spindle assembly in Drosophila oocytes begins immediately following NEB (Matthies et al. 1996), suggesting NEB somehow triggers the process. We have evidence that Subito activity is also regulated by NEB. Subito, even the unregulated form, does not bundle microtubules until after NEB. Nonetheless, spindle assembly is constrained such that microtubules only assemble around the chromosomes. On the basis of the phenotype of the subΔNT mutant, Subito may also be regulated by proximity to the chromosomes. Since spindle assembly may be initiated by overlapping microtubules rather than direct contacts with the chromosomes (see below and Karsenti and Vernos 2001), tightly regulating a protein like Subito that can bundle microtubules could be particularly important. In contrast, however, Subito is not essential for spindle assembly. There are probably several proteins or redundant mechanisms for recruiting microtubules to the spindle.
A key part of this model is that the chromosomes may not be essential for the polymerization of microtubules but may regulate the number and size of the spindles. A similar situation may occur during acentrosomal spindle formation in mammalian meiosis. Mouse oocyte microtubules can polymerize and be organized into bipolar spindles without the presence of chromosomes (Brunet et al. 1998). Furthermore, several bipolar spindles of varying sizes tended to form, indicating that chromosomes may be needed to control spindle formation and growth (Brunet et al. 1999). In mouse or Drosophila oocytes, therefore, it may be necessary to regulate the interaction of microtubules with motor proteins to occur in the vicinity of chromosomes. There are also other effects of the chromosomes. It is possible that the presence of chromosomes may promote localization of spindle pole proteins like TACC.
Interestingly, microtubules do not attach to kinetochores in mouse oocytes throughout most of prometaphase I. Instead, kinetochores are not competent to anchor and stabilize microtubule ends until the end of prometaphase I, ∼8 hr after NEB (Brunet et al. 1999). Thus, in both Drosophila and mammal oocytes, spindle assembly may be initiated by the interaction of nonkinetochore microtubules with motor proteins. Interpolar microtubules, which depend on Subito, play a critical role in organizing these bundles into the bipolar spindle. Kinetochore microtubules have a secondary role in spindle formation, being assimilated into the bipolar structure by interacting with the interpolar microtubules.
Some of our observations can be explained if a signal spreads out from the nucleus or the chromosomes themselves into the cytoplasm of stage 14 oocytes after NEB. One candidate for a signal gradient is the active form (GTP bound) of Ran, which emanates from the chromosomes and has been proposed to promote microtubule assembly around chromosomes (reviewed in Kahana and Cleveland 1999; Trieselmann and Wilde 2002; Shi and Skeath 2004; Clarke 2005). Of relevance to our studies is the observation that the addition of RCC1 or an activated form of Ran (RanG19V) stimulated microtubule assembly in the absence of chromatin (Kalab et al. 1999). Drosophila RCC1, a Ran cofactor, is found in the oocyte nucleus before and after NEB (Frasch 1991; this work) and Ran has also been suggested to have a role in meiotic spindle assembly in mouse oocytes (Cao et al. 2005) although there is evidence for RanGTP-independent pathways as well (Dumont et al. 2007). We are currently investigating if Ran signaling is involved in meiotic spindle assembly of Drosophila oocytes and if it is responsible for cytoplasmic state permissive to spindle assembly or the “organizational field” around the chromosomes or both.
Acknowledgments
We are grateful to Li Nguyen and Erica Kolibas for technical assistance and Mar Carmena, Jordan Raff, and Manfred Frasch for providing antibodies. Some stocks used in this study were obtained from the Bloomington Stock Center. This work was supported by a grant from the National Institutes of Health (GM 067142) to K.S.M.
References
- Adams, R. R., A. A. Tavares, A. Salzberg, H. J. Bellen and D. M. Glover, 1998. pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 12: 1483–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, R. R., H. Maiato, W. C. Earnshaw and M. Carmena, 2001. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell. Biol. 153: 865–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont, A. S., M. B. Braunfeld, J. W. Sedat and D. A. Agard, 1989. Large-scale chromatin structural domains within mitotic and interphase chromosomes in vivo and in vitro. Chromosoma 98: 129–143. [DOI] [PubMed] [Google Scholar]
- Blangy, A., P. Chaussepied and E. A. Nigg, 1998. Rigor-type mutation in the kinesin-related protein HsEg5 changes its subcellular localization and induces microtubule bundling. Cell Motil. Cytoskeleton 40: 174–182. [DOI] [PubMed] [Google Scholar]
- Brinkley, B. R., and J. Cartwright, Jr., 1975. Cold-labile and cold-stable microtubules in the mitotic spindle of mammalian cells. Ann. NY Acad. Sci. 253: 428–439. [DOI] [PubMed] [Google Scholar]
- Brunet, S., Z. Polanski, M. H. Verlhac, J. Z. Kubiak and B. Maro, 1998. Bipolar meiotic spindle formation without chromatin. Curr. Biol. 8: 1231–1234. [DOI] [PubMed] [Google Scholar]
- Brunet, S., A. S. Maria, P. Guillaud, D. Dujardin, J. Z. Kubiak et al., 1999. Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J. Cell. Biol. 146: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbank, K. S., A. C. Groen, Z. E. Perlman, D. S. Fisher and T. J. Mitchison, 2006. A new method reveals microtubule minus ends throughout the meiotic spindle. J. Cell. Biol. 175: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. K., Z. S. Zhong, D. Y. Chen, G. X. Zhang, H. Schatten et al., 2005. Cell cycle-dependent localization and possible roles of the small GTPase Ran in mouse oocyte maturation, fertilization and early cleavage. Reproduction 130: 431–440. [DOI] [PubMed] [Google Scholar]
- Carpenter, A. T. C., 1991. Distributive segregation: Motors in the polar wind? Cell 64: 885–890. [DOI] [PubMed] [Google Scholar]
- Cesario, J. M., J. K. Jang, B. Redding, N. Shah, T. Rahman et al., 2006. Kinesin 6 family member Subito participates in mitotic spindle assembly and interacts with mitotic regulators. J. Cell Sci. 119: 4770–4780. [DOI] [PubMed] [Google Scholar]
- Cha, B. J., L. R. Serbus, B. S. Koppetsch and W. E. Theurkauf, 2002. Kinesin I-dependent cortical exclusion restricts pole plasm to the oocyte posterior. Nat. Cell Biol. 4: 592–598. [DOI] [PubMed] [Google Scholar]
- Clarke, P. R., 2005. Cell biology. A gradient signal orchestrates the mitotic spindle. Science 309: 1334–1335. [DOI] [PubMed] [Google Scholar]
- Compton, D. A., 2000. Spindle assembly in animal cells. Annu. Rev. Biochem. 69: 95–114. [DOI] [PubMed] [Google Scholar]
- Cullen, C. F., and H. Ohkura, 2001. Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat. Cell Biol. 3: 637–642. [DOI] [PubMed] [Google Scholar]
- D'Avino, P. P., M. S. Savoian and D. M. Glover, 2005. Cleavage furrow formation and ingression during animal cytokinesis: a microtubule legacy. J. Cell Sci. 118: 1549–1558. [DOI] [PubMed] [Google Scholar]
- Dumont, J., S. Petri, F. Pellegrin, M. E. Terret, M. T. Bohnsack et al., 2007. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J. Cell. Biol. 176: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echard, A., F. Jollivet, O. Martinez, J. J. Lacapere, A. Rousselet et al., 1998. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science 279: 580–585. [DOI] [PubMed] [Google Scholar]
- Elliott, S. L., C. F. Cullen, N. Wrobel, M. J. Kernan and H. Ohkura, 2005. EB1 is essential during Drosophila development and plays a crucial role in the integrity of chordotonal mechanosensory organs. Mol. Biol. Cell 16: 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow, S. A., and D. J. Komma, 1997. Spindle dynamics during meiosis in Drosophila oocytes. J. Cell. Biol. 137: 1321–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch, M., 1991. The maternally expressed Drosophila gene encoding the chromatin-binding protein BJ1 is a homolog of the vertebrate gene Regulator of Chromatin Condensation, RCC1. EMBO J. 10: 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely, F., D. Kidd, K. Jeffers, J. G. Wakefield and J. W. Raff, 2000. D-TACC: a novel centrosomal protein required for normal spindle function in early Drosophila embryo. EMBO J. 19: 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta, K. L., J. K. Jang, E. M. Manheim, G. Subramanian and K. S. McKim, 2002. subito encodes a kinesin-like protein required for meiotic spindle pole formation in Drosophila melanogaster. Genetics 160: 1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G., and R. D. Vale, 2003. The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. J. Cell. Biol. 162: 1003–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G., F. Nedelec and R. D. Vale, 2005. Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. J. Cell. Biol. 171: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneberg, U., R. Neef, R. Honda, E. A. Nigg and F. A. Barr, 2004. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell. Biol. 166: 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, J. K., T. Rahman and K. S. McKim, 2005. The kinesin-like protein Subito contributes to central spindle assembly and organization of the meiotic spindle in Drosophila oocytes. Mol. Biol. Cell 16: 4684–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana, J. A., and D. W. Cleveland, 1999. Beyond nuclear transport. Ran-GTP as a determinant of spindle assembly. J. Cell. Biol. 146: 1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalab, P., R. T. Pu and M. Dasso, 1999. The ran GTPase regulates mitotic spindle assembly. Curr. Biol. 9: 481–484. [DOI] [PubMed] [Google Scholar]
- Karsenti, E., and I. Vernos, 2001. The mitotic spindle: a self-made machine. Science 294: 543–547. [DOI] [PubMed] [Google Scholar]
- Kramer, J., and R. S. Hawley, 2003. The spindle-associated transmembrane protein Axs identifies a membranous structure ensheathing the meiotic spindle. Nat. Cell Biol. 5: 261–263. [DOI] [PubMed] [Google Scholar]
- Kwon, M., and J. M. Scholey, 2004. Spindle mechanics and dynamics during mitosis in Drosophila. Trends Cell Biol. 14: 194–205. [DOI] [PubMed] [Google Scholar]
- Matthies, H. J., H. B. McDonald, L. S. Goldstein and W. E. Theurkauf, 1996. Anastral meiotic spindle morphogenesis: role of the non-claret disjunctional kinesin-like protein. J. Cell. Biol. 134: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar, M., and T. Misteli, 2005. Chromokinesins: multitalented players in mitosis. Trends Cell Biol. 15: 349–355. [DOI] [PubMed] [Google Scholar]
- Minestrini, G., E. Mathe and D. M. Glover, 2002. Domains of the Pavarotti kinesin-like protein that direct its subcellular distribution: effects of mislocalisation on the tubulin and actin cytoskeleton during Drosophila oogenesis. J. Cell Sci. 115: 725–736. [DOI] [PubMed] [Google Scholar]
- Moore, D. P., W. Y. Miyazaki, J. Tomkiel and T. L. Orr-Weaver, 1994. Double or nothing: a Drosophila mutation affecting meiotic chromosome segregation in both females and males. Genetics 136: 953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata, T., and N. Hirokawa, 1995. Point mutation of adenosine triphosphate-binding motif generated rigor kinesin that selectively blocks anterograde lysosome membrane transport. J. Cell. Biol. 131: 1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef, R., C. Preisinger, J. Sutcliffe, R. Kopajtich, E. A. Nigg et al., 2003. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J. Cell. Biol. 162: 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef, R., U. R. Klein, R. Kopajtich and F. A. Barr, 2006. Cooperation between mitotic kinesins controls the late stages of cytokinesis. Curr. Biol. 16: 301–307. [DOI] [PubMed] [Google Scholar]
- Nislow, C., V. A. Lombillo, R. Kuriyama and J. R. McIntosh, 1992. A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature 359: 543–547. [DOI] [PubMed] [Google Scholar]
- Raich, W. B., A. N. Moran, J. H. Rothman and J. Hardin, 1998. Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol. Biol. Cell 9: 2037–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth, P., 1998. Gal4 in the Drosophila female germline. Mech. Dev. 78: 113–118. [DOI] [PubMed] [Google Scholar]
- Salmon, E. D., and D. A. Begg, 1980. Functional implications of cold-stable microtubules in kinetochore fibers of insect spermatocytes during anaphase. J. Cell. Biol. 85: 853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupbach, T., and E. Wieschaus, 1989. Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics 121: 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, W. Y., and J. B. Skeath, 2004. The Drosophila RCC1 homolog, Bj1, regulates nucleocytoplasmic transport and neural differentiation during Drosophila development. Dev. Biol. 270: 106–121. [DOI] [PubMed] [Google Scholar]
- Skold, H. N., D. J. Komma and S. A. Endow, 2005. Assembly pathway of the anastral Drosophila oocyte meiosis I spindle. J. Cell. Sci 118: 1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer, J., and D. Kalderon, 2006. Microtubule polarity and axis formation in the Drosophila oocyte. Dev. Dyn. 235: 1455–1468. [DOI] [PubMed] [Google Scholar]
- Tao, L., A. Mogilner, G. Civelekoglu-Scholey, R. Wollman, J. Evans et al., 2006. A homotetrameric kinesin-5, KLP61F, bundles microtubules and antagonizes Ncd in motility assays. Curr. Biol. 16: 2293–2302. [DOI] [PubMed] [Google Scholar]
- Theurkauf, W. E., and R. S. Hawley, 1992. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell. Biol. 116: 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf, W. E., S. Smiley, M. L. Wong and B. M. Alberts, 1992. Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development 115: 923–936. [DOI] [PubMed] [Google Scholar]
- Trieselmann, N., and A. Wilde, 2002. Ran localizes around the microtubule spindle in vivo during mitosis in Drosophila embryos. Curr. Biol. 12: 1124–1129. [DOI] [PubMed] [Google Scholar]
- Vale, R. D., and R. J. Fletterick, 1997. The design plan of kinesin motors. Annu. Rev. Cell Dev. Biol. 13: 745–777. [DOI] [PubMed] [Google Scholar]
- Wadsworth, P., and A. Khodjakov, 2004. E pluribus unum: towards a universal mechanism for spindle assembly. Trends Cell Biol. 14: 413–419. [DOI] [PubMed] [Google Scholar]
- Walczak, C. E., I. Vernos, T. J. Mitchison, E. Karsenti and R. Heald, 1998. A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol. 8: 903–913. [DOI] [PubMed] [Google Scholar]
- Yun, M., X. Zhang, C. G. Park, H. W. Park and S. A. Endow, 2001. A structural pathway for activation of the kinesin motor ATPase. EMBO J. 20: 2611–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, C., and W. Jiang, 2005. Cell cycle-dependent translocation of PRC1 on the spindle by Kif4 is essential for midzone formation and cytokinesis. Proc. Natl. Acad. Sci. USA 102: 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]