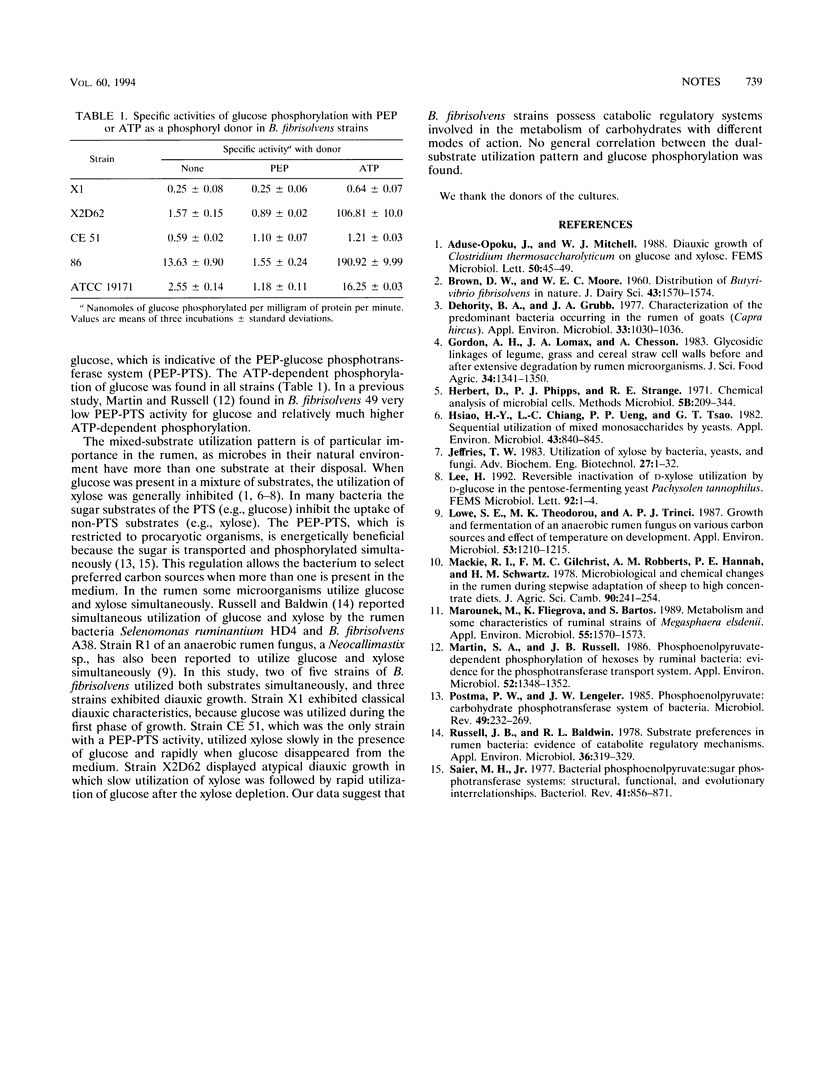
Abstract
The dual-substrate utilization pattern in cultures of five ruminal strains of Butyrivibrio fibrisolvens growing on glucose and xylose was investigated. Strains ATCC 19171 and 86 utilized glucose and xylose simultaneously. Other strains exhibited diauxic growth. Strains X1 and CE 51 exhibited classical diauxic growth in which glucose was utilized during the first phase. Strain X2D62 displayed atypical diauxic growth in which slow utilization of xylose was followed by rapid utilization of glucose after the xylose depletion. The ATP-dependent phosphorylation of glucose was found in all strains tested. The phosphoenolpyruvate-dependent phosphorylation of glucose was detected only in B. fibrisolvens CE 51.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dehority B. A., Grubb J. A. Characterization of the predominant bacteria occurring in the rumen of goats (Capra hircus). Appl Environ Microbiol. 1977 May;33(5):1030–1036. doi: 10.1128/aem.33.5.1030-1036.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao H. Y., Chiang L. C., Ueng P. P., Tsao G. T. Sequential utilization of mixed monosaccharides by yeasts. Appl Environ Microbiol. 1982 Apr;43(4):840–845. doi: 10.1128/aem.43.4.840-845.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries T. W. Utilization of xylose by bacteria, yeasts, and fungi. Adv Biochem Eng Biotechnol. 1983;27:1–32. doi: 10.1007/BFb0009101. [DOI] [PubMed] [Google Scholar]
- Lowe S. E., Theodorou M. K., Trinci A. P. Growth and fermentation of an anaerobic rumen fungus on various carbon sources and effect of temperature on development. Appl Environ Microbiol. 1987 Jun;53(6):1210–1215. doi: 10.1128/aem.53.6.1210-1215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marounek M., Fliegrova K., Bartos S. Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Appl Environ Microbiol. 1989 Jun;55(6):1570–1573. doi: 10.1128/aem.55.6.1570-1573.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Russell J. B. Phosphoenolpyruvate-dependent phosphorylation of hexoses by ruminal bacteria: evidence for the phosphotransferase transport system. Appl Environ Microbiol. 1986 Dec;52(6):1348–1352. doi: 10.1128/aem.52.6.1348-1352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol. 1978 Aug;36(2):319–329. doi: 10.1128/aem.36.2.319-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


