Abstract
Background and Purpose:
The cellular uptake of anandamide is reduced by inhibitors of fatty acid amide hydrolase (FAAH) and by agents disrupting endocytotic mechanisms. However, it is not clear if these events occur over the same time frame and if they occur to the same extent in different cells. We have therefore investigated the effects of such compounds in three cell lines of different origins using different assay incubation times and temperatures.
Experimental approach:
FAAH activity and cellular uptake of anandamide was measured using anandamide, radio-labelled either in the ethanolamine or arachidonoyl part of the molecule.
Key results:
The FAAH inhibitor URB597 inhibited the uptake of anandamide into C6 glioma, RBL2H3 basophilic leukaemia cells and P19 embryonic carcinoma cells at incubation time 4 min. However, a time-dependent and temperature-sensitive residual uptake remained after URB597 treatment. The combination of progesterone and nystatin reduced the uptake, but also decreased the amount of anandamide retained by the wells. Genistein inhibited anandamide uptake in a manner that was not additive to that of URB597. However, genistein was a potent competitive inhibitor of FAAH (Ki value 8 μM).
Conclusions and implications:
The reduction of anandamide uptake by genistein can be explained by its ability to inhibit FAAH with a potency which overlaps that for inhibition of tyrosine kinase. The FAAH- resistant but time-dependent uptake of anandamide is seen in all three cell lines studied and is thus presumably a generally occurring process.
Keywords: Endocannabinoid, anandamide, cellular uptake, fatty acid amide hydrolase, endocytosis, genistein
Introduction
In 1992, the endogenous cannabinoid, N-acylethanolamine compound, anandamide (arachidonoylethanolamide, AEA) was discovered (Devane et al., 1992). In vivo, the effects of AEA are short lasting owing to effective metabolic pathways, comprising cellular uptake and enzymatic catabolism. The enzyme fatty acid amide hydrolase (FAAH) is primarily responsible for catabolism of AEA (Deutsch and Chin, 1993), and selective FAAH inhibitors such as 3′-carbamoyl-biphenyl-3-yl-cyclohexyl-carbamate (URB597) have potentially beneficial effects in models of inflammation, inflammatory pain, anxiety and depression (Kathuria et al., 2003; Gobbi et al., 2005; Holt et al., 2005; Jayamanne et al., 2006). AEA also acts as a substrate for other enzymes such as cyclooxygenase-2 and lipoxygenases, and the products of these pathways have important biological effects of their own (see Yu et al., 1997; Kozak and Marnett, 2002; Ross et al., 2002; Matias et al., 2004).
In contrast to these well-established enzymatic pathways, the mechanism(s) responsible for the cellular accumulation of AEA is a subject of considerable discussion. Initially, it was suggested that AEA is accumulated by a process of facilitated diffusion (Di Marzo et al., 1994; Hillard et al., 1997). This is generally considered to be a property of the plasma membrane, and there is evidence that vesicles prepared from plasma membranes accumulate AEA, whereas vesicles prepared from microsomes do not (Oddi et al., 2005). However, AEA can cross membranes very rapidly indeed (Bojesen and Hansen, 2005), raising the possibility that for the incubation times often used in uptake assays (4–10 min), the processes under study are intracellular redistribution events rather than transport across the plasma membrane.
To our knowledge, there has been no success in identifying the putative AEA transporter and, indeed, the existence of such a transporter has been challenged (Patricelli and Cravatt, 2001; Glaser et al., 2003). Certainly, at short (<25 s) incubation times, the uptake of AEA shows no obvious saturability and the apparent temperature sensitivity is owing to effects on substrate availability rather than on the uptake process per se. In contrast, at longer incubation times, both saturability and temperature dependency can be seen (Glaser et al., 2003; Ligresti et al., 2004; Sandberg and Fowler, 2005; Kaczocha et al., 2006; Thors and Fowler, 2006). A number of alternative mechanisms of uptake have been proposed, of which the most well studied has been the hypothesis that FAAH regulates the transfer of AEA across the cell membrane. The change in the concentration gradient, owing to the effectiveness of FAAH to hydrolyse AEA to arachidonic acid and ethanolamine, is suggested to drive AEA from the outside and into the cell (Day et al., 2001; Deutsch et al., 2001; Glaser et al., 2003; Kaczocha et al., 2006). This is unlikely to be the only process involved as uptake has been seen in the presence of FAAH inhibitors and in preparations derived from FAAH−/− mice (Beltramo et al., 1997; Fegley et al., 2004; Ligresti et al., 2004; Ortega-Gutiérrez et al., 2004). Other possibilities include intracellular sequestration (Hillard and Jarrahian, 2000, 2003) and the presence of intracellular shuttle proteins that may or may not specifically be designated to the task of AEA transport (for a review, see Fowler, 2006). These latter processes may explain why uptake inhibitors can affect the release of AEA (Ligresti et al., 2004, Ronesi et al., 2004). In addition, there are data implicating cell membrane lipid rafts in the cellular uptake of AEA. Lipid rafts are regions of the cell membrane that are enriched in cholesterol and sphingolipids (Brown and London, 2000). Bari et al. (2005) demonstrated that cholesterol depletion by use of methyl-β-cyclodextrin dramatically reduced the rate of AEA uptake into C6 glioma cells. McFarland et al. (2004) have shown that disruption of lipid rafts by cholesterol depletors, as well as treatment with agents known to inhibit caveolae-related endocytotic processes, reduced [3H]AEA uptake in RBL2H3 cells. On the other hand, overnight preincubation with the hydroxymethylglutaryl coenzyme A inhibitor mevinolin followed by washing of the cells, did not affect the observed uptake of 100 nM AEA into P19 embryonic carcinoma cells (Sandberg and Fowler, 2005).
At first sight it can be argued that additional studies with FAAH inhibitors and disruptors of endocytotic pathways and/or lipid rafts are unlikely to add information over and above the studies described above. However, the possibility that these different processes may be operative to different extents, depending upon the cell type and/or timeframe studied has been suggested (Hillard and Jarrahian 2005; Kaczocha et al., 2006) but has not been investigated systematically, with the exception of the role played by FAAH (Glaser et al., 2003; Kaczocha et al., 2006). Thus, for example, the only published data to our knowledge, concerning the actions of the endocytotic inhibitor genistein, has been in a single cell line (RBL2H3 cells) and at a single (5 min) AEA incubation time (McFarland et al., 2004). In addition, although genistein was originally described in the literature as a ‘specific' inhibitor of tyrosine kinase (Akiyama et al. 1987), it is not known whether its effects upon the uptake of AEA are truly related to disruption of endocytotic signalling or a reflection of a separate action upon AEA metabolism. In consequence, in the present study, the effects of FAAH inhibition, nystatin + progesterone and genistein treatment upon the uptake of AEA by three different cell lines (P19 embryonic carcinoma, C6 glioma and RBL2H3 basophilic leukaemia cells) have been studied with respect to their time–dependencies and specificities of action.
Materials and methods
Culturing of cells
All cell types used were grown in 75 cm2 culturing flasks at 37°C with 5% CO2 in humidified atmospheric pressure. Passage of cells was performed twice a week and cell culture medium was changed every other day. Rat basophilic leukaemia (RBL2H3) cells (passage range 13–46) were obtained from American Type Culture Collection, Manassas, VA, USA). The cells were cultured in minimum essential medium (MEM) with Earl's salts, 2 mM L-glutamine, 15% foetal bovine serum and 100 U ml−1 penicillin + 100 μg ml−1 streptomycin. C6 rat glioma cells (passage range 13–39 were obtained from European Collection of Cell Cultures, (Porton Down, UK) The cells were cultured in F-10 Ham with 10% foetal bovine serum and 100 U ml−1 penicillin + 100 μg ml−1 streptomycin. P19 mouse embryonic carcinoma cells (passage range 9–47) were obtained from European Collection of Cell Cultures, Porton Down, UK. The cells were cultured in MEM alpha 22571 with 10% foetal bovine serum, 1% nonessential amino acids and 100 U ml−1 penicillin+100 μg ml−1 streptomycin.
Assay of [3H]AEA uptake
The method of Rakhshan et al. (2000), modified by Sandberg and Fowler (2005), was used. In 24-well culture plates, cells were plated at a density of 2 × 105 cells well−1 and incubated overnight at 37°C in an atmosphere of 5% CO2. After incubation, cells were washed once with Krebs–Henseleit–Bicarbonate (KRH) buffer (120 mM NaCl, 4.7 mM KCl, 2.2 mM CaCl2, 10 mM 4-(2-hydroxyethyl)-1-piperazineethyl-sulphonic acid, 0.12 mM KH2PO4, 0.12 mM MgSO4 in milliQ deionized water, pH 7.4) containing 1% of bovine serum albumin (BSA) and once with KRH buffer alone. When appropriate, the cells were preincubated with KRH buffer containing 0.1% of fatty acid-free BSA and selected compounds. After preincubation, [3H-arachidonoyl]AEA concentrations (50μl, in KRH buffer containing 0.1% of fatty acid-free BSA) was added to give a final volume of 400 μl, and the wells were incubated for 1, 4, 7 or 10 min as appropriate. The ethanol vehicle concentration was kept as low as possible and never exceeded 0.8 μl (0.2% v.v−1) in the assays, unless otherwise stated. Two workup protocols were used. In the ‘batch analysis protocol', the plates were directly placed on ice and each well was washed three times with 500 μl of ice-cold KRH buffer containing 1% of BSA. The buffer was then removed from the wells and 500 μl of 0.2 M NaOH was added. The plates were then incubated for 15 min at 75°C. Aliquots (300 μl) were transferred to scintillation vials. Tritium content was assayed by liquid scintillation spectroscopy with quench correction. In the ‘staged analysis protocol', the 1 min incubation plates were washed three times with 500 μl of ice-cold KRH buffer containing 1% of BSA treated this way while the 4 min samples were incubated (on a different 24-well plate). The same procedure was undertaken for the 7 and 10 min incubations. The buffer was then removed and the NaOH added as above. The protocol used is indicated in the figure legends. It should be pointed out that the process of pipetting samples takes approximately 20 s per 24-wells culture plate, and because of that the well to which substrate first was added will have a slightly longer incubation time than the last well. To compensate for this the experiments were performed in reverse order each time to get a mean value of each time point, unless otherwise stated. The ‘n' in the results refers to the number of experiments analysed, where each experiment consists of 2–4 replicates.
Calculation of temperature-adjusted added AEA concentrations
In a previous paper, we found that the concentration of AEA available for uptake was directly proportional to concentration of AEA added to the wells over the concentration range used here, but the concentration available for uptake was dependent upon the assay temperature (Thors and Fowler, 2006). With regard to the latter, it was found that for any given added concentration of AEA, the concentration available for uptake at 4°C was almost exactly half (49%) of that at 37°C. This effect of temperature on the concentration of AEA available for uptake can thus be easily compensated for by multiplying the added concentrations at 4°C by 0.49 (Thors and Fowler, 2006). In the saturation curve data undertaken at 37 and 4°C, the data at the lower temperature have been plotted both ‘as is' and after adjustment of the added AEA concentrations in this manner.
Assay of FAAH
FAAH activity was measured in rat brain membranes as described by Boldrup et al. (2004) using homogenates available in the laboratory and either [ethanolamine-1-3H]AEA or [ethanolamine-1-3H]palmitoylethanolamide as substrate. For experiments with intact cells (2 × 105 seeded into 24-well plates the day before the experiment), the method of Paylor et al. (2006) was used. Once again, the ethanol concentration did not exceed 0.8 μl 400 μl−1 assay volume for the experiments with the intact cells, whereas a higher concentration (10 μl 200 μl−1 assay) was used for the homogenates. This concentration has been shown previously by us to not affect the rate of hydrolysis of AEA by rat brain homogenates (Ghafouri et al., 2004). The two assays use different extraction protocols: in the homogenates, the reactions were stopped by addition of active charcoal in 0.5 M HCl and the aqueous phase counted for tritium content. For the intact cells, reactions were stopped by addition of methanol to the medium, followed by scraping of the cells and extraction of the reaction products using a standard chloroform–methanol separation (for further details, see Boldrup et al., 2004, Paylor et al., 2006). Blank values were from assays conducted in the absence of cells or homogenates, as appropriate.
Statistical analyses
Curve fitting, linear regression analyses and statistical comparisons were carried out with the statistical package in the GraphPad Prism computer programme (GraphPad Software Inc., San Diego, CA, USA). Kmapp values were calculated from the mean data using the direct linear plot (Eisenthal and Cornish-Bowden, 1974) analysis available in the Enzyme Kinetics v1.4 computer programme (Trinity Software, Campton, NH, USA).
Materials
Anandamide [arachidonoyl 5,6,8,9,11,12,14,15-3H] (specific activity 7. 4 TBq mmol−1; for the uptake experiments), anandamide [ethanolamine-1-3H] (specific activity 7.4 TBq mmol−1; for the FAAH assays) and palmitoylethanolamide [ethanolamine-1-3H] (specific activity 0.74 TBq mmol−1) were obtained from American Radiolabeled Chemicals Inc., St Louis, MO, USA. Unlabelled anandamide and URB597 were obtained from the Cayman Chemical Co., Ann Arbor, MI, USA. Genistein, nystatin, progesterone, fatty acid-free and normal BSA were obtained from Sigma Aldrich Inc. (St Louis, MO, USA).
Results
The effect of URB597 upon the temperature-dependent uptake of [3H]AEA uptake
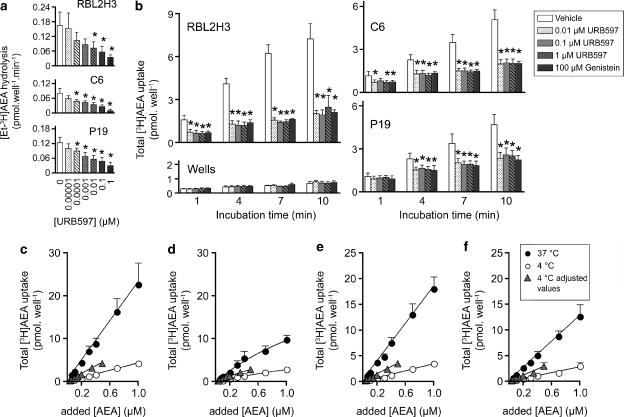
The effects of FAAH inhibitors upon the cellular uptake of AEA have been well characterized in the literature (see Introduction). However, to be able to compare the effects of FAAH inhibition with those of disruption of endocytotic processes at different times and in different cell lines, experiments with FAAH inhibitors are necessary. In consequence, we have chosen the selective FAAH inhibitor URB597 (Kathuria et al., 2003). To confirm the concentrations required for inhibition of FAAH, cells were pretreated with URB597 for 10 min before addition of ethanolamine labelled AEA ([Et-3H]AEA) and incubation for a further 20 min. (Figure 1a). As expected, a concentration of URB597 ⩾0.01 μM substantially blocked production of ethanolamine in all three cells. In a separate series using the same methodology and RBL2H3 cells, we found that the AEA hydrolytic activity remaining in the presence of 0.01, 0.1 and 1 μM URB597 was 16±5, 13±3 and 14±2% (means±s.e.m., n=6), (A Lenman and CJ Fowler, unpublished data). There was a large variation in the measured basal activity between experiments in the absence of URB597 (Figure 1a), and as a consequence there was no significant difference in activity between cell lines (one-way factorial ANOVA, F2,11=1.38, P>0.2).
Figure 1.
The role of FAAH on the uptake of [3H]AEA. (a) Effect of URB597 upon the metabolism of 100 nM [Et-3H]AEA by RBL2H3, C6 and P19 cells. The cells were preincubated with URB597 for 10 min before addition of [Et-3H]AEA and incubation for a further 20 min . Means±s.e.m., n=4. (b) Effect of URB597 and genistein upon the uptake of 100 nM [3H]AEA by either RBL2H3, C6, P19 cells or by wells alone at different incubation times. The samples were preincubated for 15 min with either vehicle or genistein and then an additional 15 min with URB597 or vehicle before addition of AEA and incubation for the times shown. The ‘batch analysis protocol' was used (see Methods section for details). Data are means±s.e.m., n=4. *P<0.05 vs the vehicle control, Dunnett's multiple comparison following significant one-way ANOVA for repeated measures at each incubation time point. For the wells at t=4 min, one value (for 1 μM URB597) was missing, and in consequence a factorial ANOVA was used for this data set. Panels (c–f) show the total uptake of [3H]AEA (means±s.e.m., n=4) by RBL2H3 cells (c,d) and P19 cells (e, f) following an incubation time of 10 min. In (d and f), the cells were treated with 1 μM URB597. The concentrations given for AEA at 37°C are those added, whereas the concentrations for AEA at 4°C are either those added, or those added × 0.49 to compensate for the effect of assay temperature on substrate availability (‘adjusted values', see Methods for details). For both RBL2H3 and P19 cells, two-way factorial ANOVA values for the data at 37°C gave significant contributions for added AEA concentration and for URB597, whereas for the data at 4°C, the contribution by URB597 was only significant for the RBL2H3 cells.
The ability of URB597 (0.01–1 μM) to inhibit the uptake of AEA into the three cell lines and to wells alone is shown in Figure 1b. In the absence of URB597, the rates of uptake (calculated from a regression analysis of the uptake at the four time points), which can be considered as the time-dependent variable, rather than initial rates of AEA uptake, were 0.64±0.10, 0.43±0.07, 0.40±0.07 and 0.04±0.01 pmol well−1 min−1 for RBL2H3, C6, P19 cells and wells alone, respectively. At 4, 7 and 10 min incubation times, the uptake was significantly reduced for all cell lines and by all URB597 concentrations, whereas the retention of the AEA by wells alone was not affected. At the 1 min time point, the uptake into the RBL2H3 and C6 cell lines was affected, whereas no significant effects were seen for the P19 cells. The time-dependent rates of uptake in the presence of 0.1 μM URB597 were 0.13±0.04, 0.13±0.03, 0.17±0.04 and 0.04±0.01 pmol well−1 min−1 for RBL2H3, C6, P19 cells and wells alone, respectively. Thus, even in the presence of URB597, a time-dependent uptake of AEA is seen. This residual time-dependent rate of uptake is essentially the same for the three cell types. Genistein (100 μM) produced the same effects as URB597 (Figure 1b).
The effect of a preincubation for 10 min with 1 μM URB597, upon the uptake of different concentrations of [3H]AEA into RBL2H3 and P19 cells, following an AEA incubation time of 10 min, is shown in Figure 1c–f. In the absence of URB597, the uptake of [3H]AEA was considerably lower at 4 than at 37°C for both RBL2H3 cells (Figure 1c) and P19 cells (Figure 1e). When the temperature-adjusted AEA concentrations were used for the data at 4°C (see Methods for details), a temperature-sensitive component was still present. URB597 significantly decreased the [3H]AEA uptake at the assay temperature of 37°C both in P19 and RBL2H3 cells (Figure 1d and f). However, even after URB597 treatment, a temperature-sensitive component of the uptake remained in both cell lines (Figure 1d and f). This indicates that the observed temperature-sensitive uptake into these cells is not solely a combination of an URB597-sensitive process and an effect of temperature upon substrate availability, but an additional process operative under the assay conditions used.
Effects of nystatin + progesterone and genistein upon AEA uptake
In their study, McFarland et al. (2004) demonstrated that the uptake of AEA into RBL2H3 cells was reduced by about half following pretreatment for 30 min with genistein (200 μM), and with 25 μg ml−1 nystatin+10 μg ml−1 progesterone. Initial experiments confirmed that these concentrations of genistein and nystatin + progesterone reduced the apparent uptake of [3H]AEA into both RBL2H3 and P19 cells, as did a lower (100 μM) concentration of genistein (Figure 1b). However, nystatin+progesterone treatment also reduced the retention of [3H]AEA by the wells by about half (in other words, to roughly the same extent in percentage terms as seen for the cells), thereby obfuscating interpretation of data obtained with this combination. The 4 min time-point data for nystatin + progesterone is shown in Figure 2 as an example, but essentially similar results were seen at 1 and 7 incubation times (data not shown). Genistein, on the other hand, did not affect the retention of [3H]AEA by the wells, and so was chosen for subsequent experiments (Figure 1b). Over a wide concentration range, and at all time points tested, genistein was found to reduce the uptake of [3H]AEA by RBL2H3 and P19 cells, whereas the retention by the wells alone was not affected to any large or consistent extent (Figure 3a). Concentrations of genistein of 20 and 200 μM were without effect on the density of the cells remaining attached to the wells (data not shown), indicating that the reduction in activity could not be attributed to a cytotoxic action of the compound. Treatment of the RBL2H3 cells with genistein also inhibited their ability to hydrolyse [3H-Et]AEA, and a pI50 value of 4.33±0.10, corresponding to an IC50 value of 47 μM, could be calculated from these data (Figure 3b).
Figure 2.
The effect of a combination of progesterone and nystatin upon the rate of AEA uptake into RBL2H3 cells, P19 cells and wells alone. The cells (or wells alone) were preincubated for 30 min with 25 μg ml−1 nystatin+10 μg ml−1 progesterone or vehicle followed by addition of 100 nM [3H]AEA and incubation for a further 4 min at 37°C. Data are means±s.e.m. (n=3; note that in this case the order of samples was not reversed between experiments). *P<0.05 vs the corresponding vehicle (0.5% ethanol) controls, two-tailed paired t-test. The values given in the figure are the progesterone + nystatin values expressed as a percentage of the vehicle control values.
Figure 3.
Effect of genistein treatment upon (a, c, d) the uptake of 100 nM [3H]AEA by cells or wells and (b) the metabolism of 100 nM [3H-Et]AEA by intact RBL2H3 cells. In panel (a), a 30 min preincubation with genistein and the ‘staged analysis protocol' was used (see Methods for details), whereas in panels (c and d) the samples were preincubated for 15 min with either vehicle or genistein and then an additional 15 min with URB597 or vehicle before addition of AEA and incubation for either 10 min (c) or 7 min (d). The inset in a shows the data for the wells with a different scale on the y-axis. In (c), the total columns represent experiments undertaken using an incubation temperature of 37°C and an incubation time of 10 min. The hatched bars enclosed within the columns represent the corresponding data for experiments undertaken at 4°C. The data shown in d were part of the same experiments as shown in Figure 1b, and the values for 0, 0.01, 0.1 URB597 and 100 μM genistein are the same as shown there for the 7 min time point. In (b), the samples were preincubated with genistein for 30 min before addition of [3H-Et]AEA and incubation for a further 20 min. Data are means±s.e.m., n=4 (n=3 for b); *P<0.05 vs control, Dunnett's multiple comparison following significant one-way ANOVA for repeated measures for each cell type (at a given temperature, when appropriate).
The effect of a combination of genistein and URB597 was investigated in three series of experiments. In the first series, the cells were preincubated with genistein for 30 min before addition of [3H]AEA and incubation for a further 10 min. URB597 or vehicle was added 15 min after the start of the genistein preincubation period. As expected, both genistein and URB597 reduced the uptake of [3H]AEA at 37°C by all three cell types tested (RBL2H3, C6 and P19 cells) without corresponding effects upon the retention by the wells alone, whereas inconsistent effects were seen at 4°C (Figure 3c). No additional inhibitory effect on the [3H]AEA uptake was seen at 37°C when genistein was incubated together with URB597 (Figure 3c). This was confirmed in the second series, where the same approach was used, but with [3H]AEA incubation times of 1, 4, 7 or 10 min (see Figure 3d for the 7 min data points). It should be noted in this series that, in the RBL2H3 cells, the 10 μM genistein concentration did not produce a maximal effect, and in this case, the combination of 0.1 μM URB597 + 10 μM genistein produced a greater effect than 10 μM genistein alone (but not 0.1 μM URB597 alone) (Figure 3d). A similar pattern was seen at incubation times of 4 and 10 min (data not shown). The effect of 10 μM genistein was maximal in the other two cell lines (Figure 3d). The experiments shown in Figure 3b and Figure 3a and d were undertaken using different incubation times and on different occasions, but there is nonetheless a reasonable correlation between effects upon uptake and AEA metabolism: thus, the mean AEA hydrolysis (as of control, 20 min incubation) for RBL2H3 cells with 5, 10, 100 and 200 μM genistein was 92, 87, 30 and 23%, whereas the mean uptake (as of control, 10 min incubation) was 68, 54, 31 and 30%, respectively.
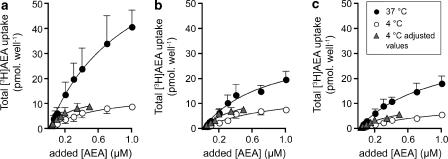
In the third series of experiments, cells were pretreated for 30 min with either vehicle, 20 μM of genistein or a combination of 20 μM genistein and 1 μM URB597 (added 15 min after the start of the genistein preincubation period) before a range of [3H]AEA concentrations were added. Experiments were performed at both 4 and 37°C. As expected, a temperature-dependent effect on the uptake was seen at the 10 min AEA incubation time used (Figure 4a). Genistein reduced the uptake, but a temperature-sensitive component remained (Figure 4b). No effect over and above that seen with genistein alone was seen for the cells pretreated with both genistein and URB597 (Figure 4c).
Figure 4.
Total uptake of [3H]AEA (means±s.e.m., n=4) by RBL2H3 cells after pretreatment either with vehicle (a), 20 μM genistein (b) or 20 μM genistein and 1 μM URB597 (c). Cells were preincubated for 30 min with the compounds before addition of [3H]AEA and incubation for a further 10 min. The concentrations given for AEA at 37°C are those added, whereas the concentrations for AEA at 4°C are either those added, or those added × 0.49 to compensate for the effect of assay temperature on substrate availability (‘adjusted values', see Methods for details). For both 4 and 37°C, two-way factorial ANOVA values for the data at 37°C gave significant contributions by both added AEA concentration and by the drug treatment.
Genistein is an effective inhibitor of FAAH
The lack of additivity of genistein and URB597 under any of the conditions tested, and the finding that the two compounds produce effects over the same time frame, would suggest that they are either operating along different parts of the same uptake pathway or, alternatively, that they share the same target. In this respect, the ability of genistein to inhibit AEA hydrolysis in the intact cells (Figure 3b) is consistent either with a reduced delivery of substrate to FAAH or with a direct inhibitory effect on the enzyme. URB597 has been shown to be very selective for FAAH (Kathuria et al., 2003, Clapper et al., 2006), but the effect of genistein upon FAAH in cell-free systems has not, to our knowledge, been tested. In consequence, this was investigated using rat brain homogenates. Preincubation with genistein for 10 min before addition of 100 nM [3H-Et]AEA, and incubation for a further 10 min, resulted in an inhibition of FAAH activity, with a significant reduction being seen at the lowest concentration tested (Figure 5a). Subsequent concentration–response curves using 2 μM [3H-Et]AEA or [3H-Et]palmitoylethanolamide as substrates (shown as insets to Figure 5a) gave pI50 values of 5.06±0.03 and 5.35±0.02, respectively, corresponding to IC50 values of 8.8 and 4.5 μM, respectively. The inhibition of AEA metabolism by genistein was not time-dependent (Figure 5b), and was competitive in nature, with a Ki value of 2.8 μM calculated from a replot of Kmapp vs [I] μM (Figure 5c). The Km value for palmitoylethanolamide (in the absence of genistein) was found, in a separate experiment, to be 2.2 μM (data not shown). Assuming a competitive interaction, this Km value, together with the IC50 value of 4.5 μM for inhibition of 2 μM palmitoylethanolamide metabolism by genistein (inset Figure 5a), corresponds to a Ki value of 2.3 μM.
Figure 5.
(a) Inhibition of 100 nM [3H-Et]AEA hydrolysis by genistein in rat brain homogenates (1.5 μg per assay). Shown are AEA hydrolysis rates following a 10 min preincubation with genistein and membranes, followed by a 10 min incubation period with AEA. Data are means±s.e.m., n=3. *P<0.01 vs control, Dunnett's multiple comparison following significant one-way ANOVA for repeated measures. The data shown in the inset are subsequent experiments (means±s.e.m., n=3) conducted on the same homogenates with either [3H-Et]AEA (2 μM) or [3H-Et]palmitoylethanolamide (2 μM). (b) Effect of preincubation time upon the inhibition of 2 μM [3H-Et]AEA hydrolysis by genistein in rat brain homogenates (3 μg per assay). The AEA incubation time was 5 min (means±s.e.m., n=3). (c) kinetics of inhibition of rat brain FAAH by genistein. In the main figure, data are means±s.e.m., n=3. The incubation time with AEA and genistein was 5 min (i.e. no genistein preincubation) and 1 μg protein per assay was used. A secondary replot of the data is shown in the inset to illustrate the competitive nature of the interaction.
The ability of nystatin and progesterone to inhibit AEA hydrolysis was also investigated using the same protocol as in the inset to Figure 5a. The compounds were without effect upon the metabolism of 2 μM [3H-Et]AEA by the rat brain homogenates. Thus, hydrolysis rates (% of control, means±s.e.m., n=3) of 99±2, 90±2 and 80±2 were found for 15, 25 and 50 μg ml−1 nystatin, respectively. The corresponding rates for progesterone (3, 10 and 30 μg ml−1) were 101±9, 95±6 and 91±7%, respectively.
Discussion
There is now good evidence that FAAH inhibitors reduce the rate of AEA uptake into a variety of (but not all) cells (Day et al., 2001; Deutsch et al., 2001; Glaser et al. 2003; Kaczocha et al., 2006; but see Beltramo et al., 1997; Ruiz-Llorente et al., 2004). In addition, evidence has been presented to suggest that disruption of membrane lipid rafts and endocytotic processes can affect AEA uptake (McFarland et al., 2004; Bari et al., 2005). In the present paper, we have reinvestigated both these processes. The original aim of the study was to determine the extent to which these processes contribute to the uptake at different time points and in different cells. However, perhaps the most important information presented here is with respect to the specificity of the agents used. There are two main findings in the present study, one perhaps expected, but one very unexpected.
FAAH-driven AEA uptake does not account for all of the accumulation of this endocannabinoid in C6, P19 and RBL2H3 cells
The effect of FAAH inhibitors upon the uptake of AEA, at different incubation time points, has been investigated previously by other groups. Deutsch et al. (2001), for example, found that the uptake of 100 nM AEA into C6 glioma and N18 neuroblastoma were reduced by the nonselective inhibitors methylarachidonoylfluorophosphonate and palmitoylsulphonyl fluoride at incubation times ⩾2 min, whereas the effect at 1 min was rather modest. The data presented were from single experiments, but in their more recent study, it was found that the selective FAAH inhibitor CAY10400 reduced the uptake of AEA into RBL2H3 cells at 45, 90 and 300 s of incubation but not at 25 s (Kaczocha et al., 2006). In our hands, a robust effect in the C6 and RBL2H3 cells was seen at all the incubation times tested, including the 1 min incubation time, whereas a 4 min incubation was required for the P19 cells. Given the hypothesis that the cellular uptake of AEA is at least in part driven by the ability of FAAH to metabolize AEA and therefore ensure a low concentration of free anandamide inside the cell compared to the medium (Day et al., 2001; Deutsch et al., 2001; Glaser et al. 2003; Kaczocha et al., 2006), an early effect of URB597 would be expected in cells like C6 and RBL2H3, that express FAAH. In contrast, HeLa cells, which do not express FAAH, show no FAAH-sensitive uptake even at an incubation time of 5 min (Day et al., 2001). The P19 cells can be considered intermediate in this respect, although their FAAH activity was not significantly different from the other cells. For both RBL2H3 and C6 cells, however, our data demonstrates that inhibition of FAAH does not remove all of the temperature-sensitive uptake seen at longer incubation times or all of the time-dependent uptake at 37°C (calculated from regression analyses of the uptakes at 1, 4, 7 and 10 min), suggesting that FAAH-driven uptake is not the only process responsible for AEA accumulation in these cells. This conclusion is entirely consistent with the findings that preparations from FAAH−/− mice can accumulate AEA (Fegley et al., 2004; Ligresti et al., 2004; Ortega-Gutiérrez et al., 2004).
Genistein reduces AEA uptake over the same time frame as seen for URB597, but this is secondary to inhibition of FAAH
In 2004, McFarland et al (2004) suggested that the uptake of AEA into RBL-2H3 cells was owing to an endocytotic process. In support of this was data demonstrating that a reduction in assay temperature, or treatment with either genistein (200 μM), N-ethylmaleimide (400 μM) or 25 μg ml−1 nystatin + 10 μg-ml−1 progesterone reduced the uptake of AEA by about half. All these treatments affect caveolin-dependent endocytotic processes via different mechanisms, and so the data, reasonably enough, were interpreted as evidence of an endocytotic mechanism of AEA uptake. However, a reduction in temperature will reduce the availability of AEA for uptake and hence the observed rate of uptake without necessarily impacting upon the uptake process itself (Thors and Fowler, 2006), and N-ethylmaleimide is an inhibitor of FAAH (Schmid et al., 1985; see Bisogno et al., 1997 for a study with RBL2H3 cell membranes), which makes interpretation of these data in terms of effects mediated by lipid rafts and/or endocytosis rather difficult. The same is true of the combination of nystatin and progesterone, which we found to be rather effective in preventing AEA retention by wells, the effect (in percentage terms) being similar to that seen upon the cellular uptake of AEA. Genistein, a tyrosine kinase inhibitor known to disrupt caveolae-related endocytosis (see Le and Nabi, 2003 and references therein), did produce robust effects, but these can be attributed to a direct action upon FAAH as (a) the effects were over the same time-course as seen with URB597 and were not additive to this compound, and (b) in cell-free preparations, genistein was a competitive inhibitor of AEA hydrolysis with a Ki value of 2.8 μM. Although there is evidence that arachidonic acid and ethanolamine produced by FAAH-catalysed hydrolysis of AEA, after its uptake, is found in caveolae-containing domains in the cell membrane in RBL2H3 cells (McFarland et al., 2004), there is no irrefutable evidence that the initial uptake requires caveolin-dependent endocytosis.
The observation that genistein inhibits FAAH is worthy of further comment. In their original study, Akiyama et al. (1987) reported that genistein inhibited the autophosphorylation of the epidermal growth factor receptor receptor in human epidermoid carcinoma A431 cell membranes with an IC50 value of 0.7 μg ml−1, which corresponds to approximately 2.6 μM, that is, close to to the Ki value for inhibition of FAAH. The compound was less effective in intact cells for both measures, autophosphorylation being inhibited with an IC50 value of 30 μg ml−1 (∼110 μM) (Akiyama et al., 1987). This value is higher than the IC50 value of 47 μM, seen in the present study, for the ability of genistein to inhibit the hydrolysis of AEA in intact RBL2H3 cells. The authors described genistein as ‘a specific inhibitor of tyrosine-specific protein kinases' in the title to their paper. Although genistein is indeed selective for tyrosine-specific protein kinase vs other kinases (Akiyama et al., 1987), the present study indicates that it is far from ‘specific' in general terms, as its potency towards FAAH overlaps that of tyrosine-specific protein kinases. The structure of genistein is unlike standard FAAH inhibitors, but the enzyme can be inhibited by phenolic compounds as disparate as propofol and cannabidiol, in both cases in an apparently competitive manner (Watanabe et al., 1998; Bisogno et al., 2001; Patel et al., 2003), so this may be a common denominator. However, structure activity studies would be needed to prove this point.
In conclusion, the present study has demonstrated that three rather different cell types (rat C6 glioma, rat RBL2H3 basophilic leukaemia and mouse P19 embryonic carcinoma cells) accumulate AEA with rather similar mechanisms inasmuch as a proportion of the uptake is sensitive to URB597/genistein. What remains to be identified is the URB597/genistein-resistant, time-dependent and temperature-sensitive component of the uptake. Given that AEA crosses biological membranes very rapidly (Bojesen and Hansen, 2005; see also Zygmunt et al., 1999 and Jerman et al., 2002, for demonstrations of the rapid activation of TRPV1 receptors, which occurs on the intracellular face of the receptor, following extracellular administration of AEA), our current working hypothesis is that the unidentified component is intracellularly localized. In this respect, Hillard and Jarrahian (2000) reported that the observed partition of 40 pmol AEA into cerebellar granule cells was about 60-fold greater than seen with 32 μM urea, which diffuses freely through cell membranes. Similarly, Rakhshan et al. (2000) concluded that the intracellular concentration of AEA (in RBL2H3 cells) ‘may exceed 100 nM' and thus be much greater than the 5 nM concentration applied to the wells. From the data shown in Figures 1b and 3c, the accumulated AEA after inhibition of FAAH that is not well associated, can be roughly estimated to be about 2–3 pmol per well after a 10 min incubation time. Assuming a volume for the C6 cells of 1 pl (Bowman et al., 1999) and approximately 2 × 105 cells well−1, this figure corresponds to an intracellular concentration of 10–15 μM. As our assays are conducted in the presence of BSA, the free extracellular concentration of AEA is much lower than the added concentration (Bojesen and Hansen, 2003), and hence the intracellular AEA is very much higher than can be explained simply in terms of free diffusion. Hillard and Jarrahian (2000) suggested that this difference could be owing to sequestration, although other possibilities include AEA-binding proteins within the cell. These would, however, need to have the binding capacity equivalent to 10-15% BSA. This calculation, based on the binding affinity of AEA for BSA (Bojesen and Hansen, 2003), is admittedly an oversimplification (see Thors and Fowler, 2006), but it does give a rough estimate of the AEA-binding capacity needed. The nature of the protein(s) providing that binding capacity still awaits elucidation. AM404 and the related uptake inhibitor UCM707 can inhibit AEA uptake in either cells lacking FAAH, preparations from FAAH−/− mice or in cells pretreated with FAAH inhibitors (Day et al., 2001; Fegley et al., 2004; Ortega-Gutiérrez et al., 2004; but see Kaczocha et al., 2006), and so it is possible that these compounds may be blocking the binding of AEA to these intracellular sites, although the ability of these compounds to inhibit the retention of AEA by wells alone (Fowler et al., 2004; Karlsson et al., 2004; Ortega-Gutiérrez et al., 2004) is a complicating factor in this respect. Hopefully, the development of irreversible inhibitors of AEA uptake (Moriello et al., 2006) will allow identification of the elusive mechanism(s) involved in the cellular uptake of AEA.
Acknowledgments
The authors would like to thank Dr. Séverine Vandevoorde for useful discussions concerning the structure of genistein and its rather unexpected interaction with FAAH. The research was supported by grants from the Swedish Research Council (Grant no. 12158, medicine), Gun and Bertil Stohne's Foundation, Konung Gustaf V's and Drottning Victorias Foundation, Stiftelsen för Gamla Tjänarinnor and the Research Funds of the Medical Faculty, Umeå University.
Abbreviations
- AEA
anandamide (arachidonoylethanolamide)
- BSA
bovine serum albumin
- FAAH
fatty acid amide hydrolase
- KRH buffer
Krebs–Henseleit–bicarbonate buffer
- URB597
3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate
Conflict of interest
The authors state no conflict of interest.
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S-i, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Bari M, Battista N, Fezza F, Finazzi-Agrò A, Maccarrone M. Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J Biol Chem. 2005;280:12212–12220. doi: 10.1074/jbc.M411642200. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Hanu L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Maurelli S, Melck D, De Petrocellis L, Di Marzo V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J Biol Chem. 1997;272:3315–3323. doi: 10.1074/jbc.272.6.3315. [DOI] [PubMed] [Google Scholar]
- Bojesen IN, Hansen HS. Binding of anandamide to bovine serum albumin. J Lipid Res. 2003;44:1790–1794. doi: 10.1194/jlr.M300170-JLR200. [DOI] [PubMed] [Google Scholar]
- Bojesen IN, Hansen HS. Membrane transport of anandamide through resealed human red blood cell membranes. J Lipid Res. 2005;46:1652–1659. doi: 10.1194/jlr.M400498-JLR200. [DOI] [PubMed] [Google Scholar]
- Boldrup L, Wilson SJ, Barbier AJ, Fowler CJ. A simple stopped assay for fatty acid amide hydrolase avoiding the use of a chloroform extraction phase. J Biochem Biophys Methods. 2004;60:171–177. doi: 10.1016/j.jbbm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Bowman CL, Yohe L, Lohr JW. Enzymatic modulation of cell volume in C6 glioma cells. Glia. 1999;27:22–31. doi: 10.1002/(sici)1098-1136(199907)27:1<22::aid-glia3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and function of sphingolipid – and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Clapper JR, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. The fatty-acid amide hydrolase inhibitor URB597 does not affect triacylglycerol hydrolysis in rat tissues. Pharmacol Res. 2006;54:341–344. doi: 10.1016/j.phrs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Day TA, Rakhshan F, Deutsch DG, Barker EL. Role of fatty acid amide hydrolase in the transport of the endogenous cannabinoid anandamide. Mol Pharmacol. 2001;59:1369–1375. doi: 10.1124/mol.59.6.1369. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Glaser ST, Howell JM, Kunz JS, Puffenbarger RA, Hillard CJ, et al. The cellular uptake of anandamide is coupled to its breakdown by fatty acid amide hydrolase (FAAH) J Biol Chem. 2001;276:6967–6973. doi: 10.1074/jbc.M003161200. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LG, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz J-C, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Eisenthal R, Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974;139:715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, et al. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci USA. 2004;101:8756–8761. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ. The cannabinoid system and its pharmacological manipulation – a review, with emphasis upon the uptake and hydrolysis of anandamide. Fund ClinPharmacol. 2006;20:549–562. doi: 10.1111/j.1472-8206.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Tiger G, Ligresti A, López-Rodíguez ML, Di Marzo V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis – a difficult issue to handle. Eur J Pharmacol. 2004;492:1–11. doi: 10.1016/j.ejphar.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Ghafouri N, Tiger G, Razdan RK, Mahadevan A, Pertwee RG, Martin BR, et al. Inhibition of monoacylglycerol lipase and fatty acid amide hydrolase by analogues of 2-arachidonoylglycerol. Br J Pharmacol. 2004;143:774–784. doi: 10.1038/sj.bjp.0705948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser S, Abumrad N, Fatade F, Kaczocha M, Studholme K, Deutsch D. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci USA. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Jarrahian A. The movement of N-arachidonoylethanolamine (anandamide) across cellular membranes. Chem Phys Lipids. 2000;108:123–134. doi: 10.1016/s0009-3084(00)00191-2. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Jarrahian A. Cellular accumulation of anandamide: consensus and controversy. Br J Pharmacol. 2003;140:802–808. doi: 10.1038/sj.bjp.0705468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Jarrahian A. Accumulation of anandamide: evidence for cellular diversity. Neuropharmacology. 2005;48:1072–1078. doi: 10.1016/j.neuropharm.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of narachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem. 1997;69:631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- Holt S, Comelli F, Costa B, Fowler CJ. Inhibitors of fatty acid amide hydrolase reduce carrageenan-induced hind paw inflammation in pentobarbital-treated mice: comparison with indomethacin and possible involvement of cannabinoid receptors. Br J Pharmacol. 2005;146:467–476. doi: 10.1038/sj.bjp.0706348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerman JC, Gray J, Brough SJ, Ooi L, Owen D, Davis JB, et al. Comparison of effects of anandamide at recombinant and endogenous rat vanilloid receptors. Br J Anaesth. 2002;89:882–887. doi: 10.1093/bja/aef281. [DOI] [PubMed] [Google Scholar]
- Kaczocha M, Hermann A, Glaser ST, Bojesen IN, Deutsch DG. Anandamide uptake is consistent with rate-limited diffusion and is regulated by the degree of its hydrolysis by fatty acid amide hydrolase. J Biol Chem. 2006;281:9066–9075. doi: 10.1074/jbc.M509721200. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Påhlsson C, Fowler CJ. Reversible, temperature-dependent, and AM404-inhibitable adsorption of anandamide to cell culture wells as a confounding factor in release experiments. Eur J Pharm Sci. 2004;22:181–189. doi: 10.1016/j.ejps.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nature Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Marnett LJ. Oxidative metabolism of endocannabinoids. Prostaglandins Leukot Essent Fatty Acids. 2002;66:211–220. doi: 10.1054/plef.2001.0359. [DOI] [PubMed] [Google Scholar]
- Le PU, Nabi IR. Distinct caveolae-mediated endocytic pathways target the golgi apparatus and the endoplasmic reticulum. J Cell Sci. 2003;116:1059–1071. doi: 10.1242/jcs.00327. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Morera E, Van Der Stelt M, Monory K, Lutz B, Ortar G, et al. Further evidence for the existence of a specific process for the membrane transport of anandamide. Biochem J. 2004;380:265–272. doi: 10.1042/BJ20031812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Chen J, De Petrocellis L, Bisogno T, Ligresti A, Fezza F, et al. Prostaglandin ethanolamides (prostamides): in vitro pharmacology and metabolism. J Pharmacol Exp Ther. 2004;309:745–757. doi: 10.1124/jpet.103.061705. [DOI] [PubMed] [Google Scholar]
- McFarland MJ, Porter AC, Rakhshan FR, Rawat DS, Gibbs RA, Barker EL. A role for caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide. J Biol Chem. 2004;279:41991–41997. doi: 10.1074/jbc.M407250200. [DOI] [PubMed] [Google Scholar]
- Moriello AS, Balas L, Ligresti A, Cascio MG, Duran M, Morera E, et al. Development of the first potential covalent inhibitors of anandamide cellular uptake. J Med Chem. 2006;49:2320–2332. doi: 10.1021/jm051226l. [DOI] [PubMed] [Google Scholar]
- Oddi S, Bari M, Battista N, Barsacchi D, Cozzani I, Maccarrone M. Confocal microscopy and biochemical analysis reveals spatial and functional separation between anandamide uptake and hydrolysis in human keratinocytes. Cell Mol Life Sci. 2005;62:386–395. doi: 10.1007/s00018-004-4446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Gutiérrez S, Hawkins EG, Viso A, López-Rodríguez ML, Cravatt BF. Comparison of anandamide transport in FAAH wild-type and knockout neurons: evidence for contributions by both FAAH and the CB1 receptor to anandamide uptake. Biochemistry. 2004;43:8184–8190. doi: 10.1021/bi049395f. [DOI] [PubMed] [Google Scholar]
- Patel S, Wohlfeil ER, Rademacher DJ, Carrier EJ, Perry LJ, Kundu A, et al. The gerneral anesthetic propofol increases brain N-arachidonoylethanolamine (anandamide) content and inhibits fatty acid amide hydrolase. Br J Pharmacol. 2003;139:1005–1013. doi: 10.1038/sj.bjp.0705334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricelli MP, Cravatt BF. Proteins regulating the biosynthesis and inactivation of neuromodulatory fatty acid amides. Vitamins Hormones. 2001;62:95–131. doi: 10.1016/s0083-6729(01)62002-8. [DOI] [PubMed] [Google Scholar]
- Paylor B, Holt S, Fowler CJ. The potency of the fatty acid amide hydrolase inhibitor URB597 is dependent upon the assay pH. Pharmacol Res. 2006;54:481–485. doi: 10.1016/j.phrs.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Rakhshan F, Day TA, Blakely RD, Barker EL. Carrier-mediated uptake of the endogenous cannabinoid anandamide in RBL2H3 cells. J Pharmacol Exp Ther. 2000;292:960–967. [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Craib SJ, Stevenson LA, Pertwee RG, Henderson A, Toole J, et al. Pharmacological characterization of the anandamide cyclooxygenase metabolite: prostaglandin E2 ethanolamide. J Pharmacol Exp Ther. 2002;301:900–907. doi: 10.1124/jpet.301.3.900. [DOI] [PubMed] [Google Scholar]
- Ruiz-Llorente L, Ortega-Gutiérrez S, Viso A, Sánchez MG, Sánchez AM, Fernández C, et al. Characterization of an anandamide degradation system in prostate epithelial PC-3 cells: synthesis of new transporter inhibitors as tools for this study. Br J Pharmacol. 2004;141:457–467. doi: 10.1038/sj.bjp.0705628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg A, Fowler CJ. Measurement of saturable and non-saturable components of anandamide uptake into P19 embryonic carcinoma cells in the presence of fatty acid-free bovine serum albumin. Chem Phys Lipids. 2005;134:131–139. doi: 10.1016/j.chemphyslip.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Schmid PC, Zuzarte-Augustin ML, Schmid HHO. Properties of rat liver N-acylethanolamine amidohydrolase. J Biol Chem. 1985;260:14145–14149. [PubMed] [Google Scholar]
- Thors L, Fowler CJ. Is there a temperature-dependent uptake of anandamide into cells. Br J Pharmacol. 2006;149:173–181. doi: 10.1038/sj.bjp.0706831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Ogi H, Nakamura S, Kayano Y, Matsunaga T, Yoshimura H, et al. Distribution and characterization of anandamide amidohydrolase in mouse brain and liver. Life Sci. 1998;62:1223–1229. doi: 10.1016/s0024-3205(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H-H, Sørgård M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]