Abstract
Background and purpose:
We have previously shown that melatonin inhibits bradykinin-induced NO production by endothelial cells in vitro. The purpose of this investigation was to extend this observation to an in vivo condition and to explore the mechanism of action of melatonin.
Experimental approach:
RT-PCR assays were performed with rat cultured endothelial cells. The putative effect of melatonin upon arteriolar tone was investigated by intravital microscopy while NO production by endothelial cells in vitro was assayed by fluorimetry, and intracellular Ca2+ measurements were assayed by confocal microscopy.
Key results:
No expression of the mRNA for the melatonin synthesizing enzymes, arylalkylamine N-acetyltransferase and hydroxyindole-O-methyltransferase, or for the melatonin MT2 receptor was detected in microvascular endothelial cells. Melatonin fully inhibited L-NAME-sensitive bradykinin-induced vasodilation and also inhibited NO production induced by histamine, carbachol and 2-methylthio ATP, but did not inhibit NO production induced by ATP or α, β-methylene ATP. None of its inhibitory effects was prevented by the melatonin receptor antagonist, luzindole. In nominally Ca2+-free solution, melatonin reduced intracellular Ca2+ mobilization induced by bradykinin (40%) and 2-methylthio ATP (62%) but not Ca2+ mobilization induced by ATP.
Conclusions and implications:
We have confirmed that melatonin inhibited NO production both in vivo and in vitro. In addition, the melatonin effect was selective for some G protein-coupled receptors and most probably reflects an inhibition of Ca2+ mobilization from intracellular stores.
Keywords: endothelial cell, nitric oxide, melatonin, calcium, ATP, bradykinin, histamine, intravital microscopy, confocal microscopy
Introduction
Melatonin (N-acetyl-5-methoxytryptamine), the hormone produced by the pineal gland, acts as an endocrine transducer of photoperiodic information, although extra-pineal sources account for paracrine and/or autocrine actions (Pontes et al., 2006). Endothelial cells were previously considered as a putative extra-pineal source of melatonin (Kvetnoy, 1999), although the key enzyme involved in its synthesis, namely arylalkylamine N-acetyltransferase (AA-NAT) and also hydroxyindole-O-methyltransferase (HIOMT), have not yet been investigated. Melatonin modulates endothelial cell function in vitro by inhibiting bradykinin-induced nitric oxide (NO) production (Tamura et al., 2006) and in vivo by inhibiting neutrophil rolling and adherence (Lotufo et al., 2001).
In mammals, melatonin targets three distinct high-affinity melatonin receptors (MT1, MT2 and MT3). MT1 and MT2 melatonin receptors belong to the heptahelical G-protein coupled receptor family, whereas the MT3 receptor is known as the enzyme quinone oxidoreductase 2 (Dubocovich and Markowska, 2005). Despite some controversy, melatonin has also been described as a ligand for cytoplasmic proteins (Benitez-King et al., 1993) and for a family of orphan nuclear hormone receptors (retinoic acid receptor-related orphan receptor/retinoid Z receptor) (Boutin et al., 2005).
Putative melatonin receptors in arteries were first identified in rat brain and tail by autoradiography using 2-[125I]-iodomelatonin (Viswanathan et al., 1990; Seltzer et al., 1992; Capsoni et al., 1994). More recently, functional assays in vitro suggested that melatonin is involved in the control of vasomotor tone and acts via receptors located in the myocytes (Masana et al., 2002) and/or in the endothelium (Geary et al., 1998; Yang et al., 2001). In another experimental model, melatonin was shown to inhibit the rolling of leukocytes on the venular endothelial layer in vivo by stimulating MT2 melatonin receptors (Lotufo et al., 2001). Antisense [33P]-labeled oligonucleotide probe specific for MT2 melatonin receptors hybridized to the three layers of rat caudal artery (tunica adventitia, media and intima), suggesting that endothelial cells express MT2 melatonin receptors (Masana et al., 2002). A previous pharmacological analysis of the inhibitory effect of melatonin on bradykinin-induced NO production in cultured endothelial cells precludes mediation by MT2 melatonin receptors, as it is not inhibited by the selective antagonist 4-phenyl-2-propionamidotetralin (4P-PDOT) (Tamura et al., 2006).
In resting endothelial cells, the constitutive endothelial nitric oxide synthase (eNOS) is located in the plasmalemmal caveolae associated with the inhibitory protein caveolin. Elevation of intracellular Ca2+ disrupts the caveolin/eNOS complex, thereby favoring eNOS translocation from the plasma membrane and its subsequent activation, ultimately resulting in the conversion of its substrate L-arginine into L-citrulline and NO (Dudzinski et al., 2006). In some vascular beds, the endothelium-dependent vascular relaxation induced by laminar shear stress or Ca2+ mobilization through G-protein coupled receptors is mediated by NO (Cocks, 1996). In addition, it was recently shown that eNOS also regulates vascular permeability, linking this enzyme to a key event of the inflammatory process (Hatakeyama et al., 2006).
The purpose of this investigation was to determine if melatonin could inhibit endothelial production of NO in vivo and to explore the mechanism of action of the pineal hormone with regard to modulation of NO activity. Firstly, we determined whether endothelial cells express the mRNA for two enzymes involved in the biosynthesis of melatonin, AA-NAT and HIOMT and also for MT2 melatonin receptors. Then, we evaluated the effect of melatonin on other agonists such as carbachol, histamine, adenosine triphosphate (ATP) and analogues, and finally we investigated the relevance of intracellular Ca2+ mobilization to the melatonin-induced decrease in NO production.
We report here that endothelial cells from rat microcirculation did not express the enzymes, AA-NAT and HIOMT or MT2 melatonin receptors. In functional assays in vivo, melatonin inhibited Nϖ-nitro-L-arginine methyl ester (L-NAME)-sensitive mesenteric arteriolar vasodilation induced by bradykinin. The NO production induced by histamine and carbachol in vitro was also inhibited by melatonin, as observed previously with bradykinin (Tamura et al., 2006); however, melatonin did not inhibit the effect of ATP. Two other agonists that activate ligand-gated ion channel P2X receptors (α,β-methylene ATP) and metabotropic P2Y receptors (2-methylthio-adenosine triphosphate (2-methylthio ATP)) also elicited NO production by endothelial cells, but only the effect of the latter agonist was inhibited by melatonin. The inhibitory effect of melatonin on these agonists of G-protein coupled receptors seems to be partly related to an inhibition of Ca2+ mobilization from intracellular stores.
Methods
All animal procedures were performed according to approved institutional protocols and in accordance with recommendations for the proper use and care of laboratory animals. Animals were kept under a light/dark cycle of 12/12 h and had access to water and food ad libitum.
Endothelial cell culture
Primary cultures of microvascular endothelial cells were obtained from rat cremaster muscle according to a method described previously (Tamura et al., 2006). Male Wistar rats weighing about 250 g were anesthetized by ether inhalation and killed by decapitation. The cremaster muscle was isolated, washed with phosphate saline solution (mM: NaCl 125, Na2HPO4 2, NaH2PO4 2 and KCl 5) and cut into pieces of approximately 2 × 2 mm. Two pieces were placed into a 24-well culture plate, Dulbecco's Modified Eagle Medium (DMEM) supplemented with gentamicin (40 mg l−1) and 20% fetal bovine serum was added, and the cells were cultured in a humidified incubator at 37°C with 5% CO2 for 48 h. After this period, the explants were removed and the medium was changed every 48 h. Cultured cells were characterized using flow cytometry analysis as described by Lotufo et al. (2006), which showed only one population of cells positively labeled with the monoclonal antibody selective for platelet-endothelial cell adhesion molecule 1 (PECAM-1), a classical marker of endothelial cells (data not shown).
Total RNA isolation and quantification
Total RNA was extracted from confluent primary cultured rat endothelial cells with TRIzol and chloroform–isopropanol – 75% ethanol (in diethyl pyrocarbonate-treated water) according to the manufacturer's instructions. Each endothelial sample consisted of two wells of a 24-well plate culture from different cultures. Rat pineal glands obtained from animals killed during the light phase of the day were used as controls (de Almeida-Paula et al., 2005). The optical density (OD) of each sample was determined using an ultraviolet (UV)-visible spectrophotometer (GeneQuant, Amersham Pharmacia Biotech, Cambridge, UK) for quantification of RNA content. The RNA samples used in the following steps had OD λ260/λ280 ratios ranging from 1.8 to 2.0.
Reverse transcription
Single-stranded complementary DNA (cDNA) was generated from 0.5 μg of total RNA using 1 μl (50 ng) of random primers and 1 μl of 10 mM deoxyribonucleotide triphosphate mix (dNTP) (65°C, 5 min), followed by the addition of 4 μl of 5 × polymerase chain reaction (PCR) buffer (supplied with transcriptase) and 2 μl of 0.1 M dithiothreitol (25°C for 2 min), and finally 1 μl of SuperScript II reverse transcriptase (RT) (200 U) in a final volume of 20 μl. The RT mixture was incubated (25°C, 10 min; 42°C, 50 min and 70°C, 15 min) to promote cDNA synthesis. cDNA samples were stored at −20°C for up to 1 week.
Real time RT-PCR for AA-NAT and HIOMT
Reactions were performed in a 25 μl final volume using 1 μl cDNA or water (negative control), 300 nM (Aa-nat and hiomt) or 50 nM (18S) of sense and antisense primers and 12.5 μl 2 × iQ SYBR Green Supermix (including iTaq DNA polymerase) (Bio-Rad Laboratories, Hercules, CA, USA). Real time-PCR amplification and quantification were performed with an i-Cycler thermal cycler (Bio-Rad Laboratories, CA, USA) as follows: denaturation for 7 min at 95°C, followed by 40 cycles of 10 s at 95°C and 1 min at 60°C. Rat pineal glands were used as a positive control for Aa-nat and hiomt mRNA expression. Therefore, rat pineal glands were kept in BGJb medium supplemented with 2 mM glutamine, 100 U ml−1 penicillin and 10 μg ml−1 streptomycin for 48 h (37°C, 95% O2/5% CO2), and in the last 5 h glands were treated with noradrenaline (0.1 μM at 37°C and 95% O2/5% CO2) as described previously (Fernandes et al., 2006).
Primers for rat Aa-nat, hiomt and 18S ribosomal RNA were synthesized by Biosource International Inc. (Camarillo, CA, USA). Aa-nat: forward: 5′-AGCGCGAAGCCTTTATCTCA-3′, reverse: 5′-AAGTGCCGGATCTCATCCAA-3′; hiomt: forward: 5′-AGCGCCTGCTGTTCATGA-3′, reverse: 5′-GGAAGCGTGAGAGGTCAA-3′; 18S: forward: 5′-CGTCTACCACATCCAAGGAA-3′, reverse: 5′-GCTGGAATTTACCGCCGGCT-3′.
The cycle threshold (CT) determination was performed considering the threshold as close as possible to the base of the exponential phase. The mean CT value of the pineal gland was used as a calibrator, and the relative amount of RNA was calculated using the equation 2−ΔΔCT, where ΔΔCT is the ΔCT (pineal gland) – ΔCT (noradrenaline-treated pineal gland or endothelial cells) and ΔCT is CT (test gene) – CT (control gene; 18S RNA).
RT-PCR for MT melatonin receptors
Oligonucleotide primers specific for MT2 melatonin receptors were designed as described previously (de Almeida-Paula et al., 2005) and consisted of the sequence: 5′-CATCCACTTCCTCCTTCCAA-3′ (forward) and 5′-TGCAAGGCCAATACAGTTGA-3′ (reverse) (predicted size 201 bp, primer locations 123–142 bp and 323–304 bp). In the PCR, 1 μl of cDNA, 0.3 μl of Platinum Taq DNA polymerase (5 U μl−1), 0.5 μl of 10 mM dNTP mix, 2 μl of each primer (10 pmol μl−1), 2.5 μl of 10 × PCR buffer and 1.5 μl of 50 mM MgCl2 (all Invitrogen, Carlsbad, CA, USA) were used in a final volume of 25 μl. The PCR reaction started with 2 min denaturation at 95°C followed by 35 cycles of 30 s denaturation at 95°C, 45 s annealing at 60°C and 1 min extension at 72°C, and ended with a final cycle of 10 min at 72°C. The PCR was performed with the Eppendorf Master Cycler gradient in duplicate. A 10 μl aliquot of each sample (from endothelial cells, pineal gland and negative control) was run on a 1.8% agarose gel stained with 1% ethidium bromide for approximately 25 min at 100 V. The gel was visualized with an UV transilluminator and photographed using a UV gel electrophoresis camera (de Almeida-Paula et al., 2005).
High performance liquid chromatography measurements
To assess if endothelial cells produce melatonin, we incubated endothelial cells in culture, with the melatonin precursor, N-acetylserotonin (20 μM), for 5 h (37°C, 95% O2/5% CO2). Controls were performed by incubating rat pineal glands with 20 μM N-acetylserotonin for the same period (final volume 200 μl). Melatonin present in the supernatant was measured by high performance liquid chromatography according to Ferreira et al. (1994). Briefly, the indoleamine was separated on a Resolve C18 reversed-phase column (5 μm, 150 × 3.9 mm internal diameter from Waters, Milford, MA, USA). The chromatographic system (Shimadzu, Kyoto, Japan) was isocratically operated with the following mobile phase: 0.1 M citric acid, 0.15 mM ethylenediaminetetraacetic acid, 25% methanol, pH 3.7 at a flow rate of 0.7 ml min−1). The detector potential was adjusted to +0.90 V (vs Ag/AgCl reference electrode). The supernatant (20 μl) was injected into the chromatographic system. Using a calibration curve with melatonin, the lower limit of detection of melatonin with our system was 0.25 ng μl−1.
Intravital microscopy
Intravital microscopy assays were performed according to methods described previously (Lotufo et al., 2001). Briefly, male Wistar rats (about 200 g) were anesthetized with sodium pentobarbital (65 mg/kg intraperitoneal), the abdomen was carefully opened with a small midline incision and the rat was placed on its right side. Thereafter, a segment of the mid-jejunum was removed and placed over an optically clear viewing platform to be transilluminated. The animals were kept on a special board thermostatically controlled at 37°C. The exposed mesentery was kept moist and warm by irrigating the tissue with warmed Ringer–Locke solution (mM: NaCl 154, KCl 5.6, CaCl2 2, NaHCO3 6 and glucose 5; pH 7.2–7.4) containing 1% gelatin. Transilluminated images were obtained with an Axioplan optical microscopy (Carl Zeiss, New York, NY, USA) equipped with 5.0/0.30 × plan-neofluar or 10.0/0.25 × Achroplan longitudinal distance objectives/numeric aperture and 1.0 × , 1.25 × or 1.60 × optovar. The images were captured by a video camera (ZVS, 3C75DE, Carl Zeiss), transmitted simultaneously to a TV monitor and a computer and recorded on videotape. Analysis of digital images on the computer monitor was performed with KS 300 software (Kontron ElektroniK, Hallbergmoos, Germany).
After choosing a field with a continuous blood flow, the inner diameter of a third-order arteriole (mean value 19.2 μm) was measured and defined as a basal diameter. Changes in the arteriole diameter induced by pharmacological treatment were determined and expressed as percent variation from basal diameter. All drugs were topically added to the vessel in a standard volume of 10 μl. Bradykinin (1 μM) was added in the absence or in the continuous presence of either L-NAME (500 μM) or melatonin (1 nM) added 2 min previously.
Fluorescence measurements
Nitric oxide measurements
Endothelial NO production was determined by spectrofluorimetry as described previously (Tamura et al., 2006). Briefly, NO released by endothelial cells for a 30-min period was measured using fluorimetric assays according to methods described elsewhere (Nakatsubo et al., 1998). Cultured endothelial cells were incubated with 2 μM 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA, Molecular Probes, Eugene, Oregon, USA), which is very selective for NO (Balcerczyk et al., 2005) and reacts with NO to yield fluorescent triazolofluorescein (Kojima et al., 1999). Primary endothelial cells were seeded onto 96-well culture plates, grown to confluence and then washed with a physiological solution (mM: NaCl 140, KCl 5, MgCl2 1, CaCl2 2, glucose 5 and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 5; pH 7.4) supplemented with L-arginine (1 mM) (Kimura et al., 2004). Cells were then incubated at 37°C for 30 min with DAF-FM in the absence (basal) or presence of histamine (10 μM), carbachol (100 μM), ATP (100 μM), α,β-methylene ATP (100 μM) or 2-methylthio ATP (30 μM). Alternatively, these drugs were added in the presence of melatonin (1 nM) for 30 min. Experiments using the nonselective MT receptor antagonist, luzindole (N-acetyl-2-benzyltryptamine) (10 μM), were preceded by a preincubation period of 60 min at 37°C followed by the addition of melatonin and the agonists for another 30 min in the continued presence of the antagonist. The cellular supernatants (300 μl) were collected, added to a white microplate and the fluorescence was measured with a fluorimeter (Victor, Perkin-Elmer, Wellesley, MA, USA) using filters at 488 and 514 nm for excitation and emission, respectively. Because melatonin, DAF-FM and luzindole are all photosensitive, these experiments were performed in the dark.
Intracellular Ca2+ measurements
Relative changes in endothelial cytosolic Ca2+ were determined with a confocal laser-scanning microscope (LSM 500, Carl Zeiss) using the fluo-3 AM fluorescent probe (Molecular Probes) (Tamura et al., 2006). Briefly, endothelial cells subcultured for up to two passages were seeded onto glass coverslips and allowed to grow for 48 h. The coverslip was mounted on the stage of an inverted microscope equipped with a 40 × oil-immersion objective (Plan-Neofluar, NA=1.3; Carl Zeiss). Typically, a field with 5–7 cells was randomly chosen and imaged. Cells were incubated in the dark with 5 μM fluo-3 AM diluted in a physiological solution (mM: NaCl 145, KCl 5, MgCl2 1, CaCl2 2, glucose 10 and HEPES 10) adjusted to pH 7.4 with NaOH, for 50 min at room temperature.
Following this period, the cells were washed four times with a Ca2+-free solution in which CaCl2 was omitted and 0.1 mM ethylene glycol-bis(β-aminoethyl ether) tetraacetic acid (EGTA) was added. The absence of residual extracellular calcium was confirmed by stimulating the cells with α,β-methylene ATP (100 μM), a selective agonist of ionotropic P2X receptors. Cells were then incubated with nominally Ca2+-free solution (no EGTA added) in which bradykinin (0.1 μM), ATP (100 μM) or 2-methylthio ATP (30 μM) was added in the absence or presence of melatonin (1 nM) preincubated for 1 min (Tamura et al., 2006). The dye was excited at 488 nm with an argon laser and the emitted fluorescence was measured at 515–530 nm. Fluorescent images were obtained every 0.2 s as a 512 × 512-pixel frame, and all other settings including pinhole, scanning speed and laser power remained the same for all experiments.
Data analysis and statistical procedures
Data from intravital microscopy are expressed as percent variation from basal arteriolar diameter. The variation of intracellular Ca2+ induced by agonists was measured for 3 min. Afterward, the fluorescence of each cell in the plate was measured and a mean value of the plate was determined. This value, expressed as arbitrary units, was used to calculate the amplitude (peak) of the response for each plate (GraphPad Prism 4.0 software; San Diego, CA, USA). Different cell cultures (4–7) were used in this protocol. NO measurements were also expressed as arbitrary units and each experimental condition was evaluated in at least three different cell cultures. Data are presented as mean±s.e.m. Unpaired Student's t-test or one-way analysis of variance (ANOVA) followed by Newman–Keuls post hoc test were performed for determining the significance of the differences between experimental conditions, with P<0.05 considered significant.
Drugs, chemicals, reagents and other materials
The PECAM-1 monoclonal antibody conjugated with R-phycoerythrin (anti-rat CD31) was purchased from BD Pharmingen (Franklin Lakes, NJ, USA).
Melatonin, bradykinin, histamine, ATP, 2-methylthio ATP, α,β-methylene ATP, EGTA and HEPES were purchased from Sigma Chemical Co. (St Louis, MO, USA); luzindole (N-acetyl-2-benzytryptamine), L-NAME and carbachol were acquired from Tocris (Ballwin, MO, USA). DMEM, fetal bovine serum and gentamicin reagent solution were purchased from GIBCO BRL Products (Grand Island, NY, USA). DAF-FM DA and fluo-3 AM were obtained from Molecular Probes (Eugene). Sodium pentobarbital was purchased from Cristália (São Paulo, Brazil).
Stock solutions were prepared in 5% acetic acid (10−2 M, bradykinin), deionized water (10−2 M, histamine, L-NAME and carbachol), 100% dimethylsulphoxide (DMSO) (10−3 M, DAF-FM, fluo-3 AM), 100% ethanol (10−2 M, luzindole) or 20% ethanol (10−2 M, melatonin) and stored at −20°C. The subsequent dilutions were made with one of the described buffered physiological solutions depending on the protocol used. ATP, 2-methylthio ATP and α,β-methylene ATP solutions (10−2 M) were prepared daily using the respective buffered physiological solutions. The final concentration of the solvent was ⩽0.1% (v/v) (except for DAF-FM, 0.2% DMSO) and had no effect on the experiments.
Results
RT-PCR studies
Real time RT-PCR assays revealed, as expected, the presence of mRNA for the enzyme AA-NAT in rat pineal gland, which was used as a relative calibrator in this study (Fernandes et al., 2006). The analysis of the data using the CT method revealed a ΔCT value of 20.27±0.87 (n=3), 13.63±0.48 (n=3) and 25.2±0.73 (n=5) for pineal gland (calibrator), pineal gland stimulated with 0.1 μM noradrenaline (positive control) and endothelial cells, respectively. The calculated ΔΔCT values for these samples were −6.64±0.48 (n=3) and 4.93±0.73 (n=5) for pineal glands stimulated with noradrenaline and endothelial cells, respectively, yielding according to the equation 2−(ΔΔCT) a 99.7-fold relative Aa-nat mRNA expression in treated pineal gland (range 71.5–139.1) and 0.033-fold in endothelial cells (range 0.019–0.054). A similar assessment of hiomt mRNA expression disclosed a 0.015-fold expression in endothelial cells (range 0.0078–0.0301; n=5) in relation to the calibrator. Therefore, endothelial cells from three different cultures did not express the enzymes involved in the biosynthetic pathway for melatonin. Furthermore, no PCR product was detectable when the RT-PCR steps were carried out with no added template, indicating that all reagents were free of target sequence contamination. The production of melatonin by endothelial cells or pineal glands incubated with N-acetylserotonin (20 μM) for 5 h was used as a test of HIOMT activity. Pineal glands produced 113±6 ng per well (n=5) of melatonin after 5 h of incubation, whereas no detectable melatonin was produced by endothelial cells. Taking into account the determination of hiomt gene expression and HIOMT enzyme activity, we concluded that in this experimental model endothelial cells did not synthesize melatonin.
In another experimental protocol, RT-PCR failed to detect MT2 receptor mRNA transcript in cultured rat endothelial cells (samples consisting of two different cultures obtained from two animals), whereas a transcript of the expected product size (202 bp) was detected in the positive control (non-treated pineal glands) (Figure 1) (de Almeida-Paula et al., 2005). The identity of the amplified fragment from the pineal gland using the primers mentioned above was confirmed previously by DNA sequence analysis (de Almeida-Paula et al., 2005). As stated previously, no PCR product was detected when the PCR steps were carried out without cDNA.
Figure 1.
RT-PCR analysis of MT2 melatonin receptor mRNA expression in pineal gland (P; positive control) and two separate cultures of endothelial cells (E1, E2). N=negative control (no template added); MW=molecular weight standards. RT-PCR transcripts were obtained in two independent PCR steps and separated by electrophoresis in agarose gel (see Methods).
Intravital microscopy
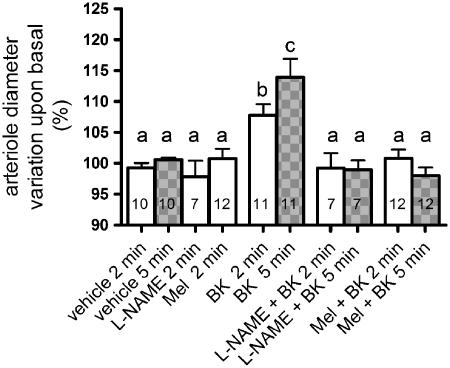
Addition of bradykinin (1 μM for 2 and 5 min) induced a significant increase, over basal values, in the arteriolar diameter of third-order arterioles of rat mesentery, as measured by intravital microscopy (Figure 2). The addition of vehicle (phosphate buffered saline) caused no alteration in relation to basal diameter (P=0.36). Bradykinin-induced vasodilation observed 2 or 5 min after addition was inhibited by prior treatment with L-NAME (500 μM for 2 min), which indicates that the agonist effect relies upon NO production. Furthermore, pre-incubation of the tissue with melatonin (1 nM) for 2 min completely abolished the vasodilator effect of bradykinin, at both time points measured (Figure 2).
Figure 2.
Effects of melatonin on L-NAME-sensitive, bradykinin-induced, rat arteriolar vasodilation. Arteriolar diameter (mean basal diameter: 19.2 μm) was measured 2 (clear bars) and 5 min (grey bars) after vehicle, bradykinin (BK, 1 μM), L-NAME (500 μM) or melatonin (Mel, 1 nM). The effect of BK was determined in the absence or presence of L-NAME or Mel which were added 2 min before and maintained throughout the exposure to BK. Data are expressed as mean±s.e.m. of the individual measurements indicated in the columns. Different letters indicate significant differences between the experimental conditions (P<0.05; ANOVA, followed by Newman–Keuls test). The number of rats per group was vehicle (7), BK (8); L-NAME (2) and Mel/BK (6).
Nitric oxide measurements
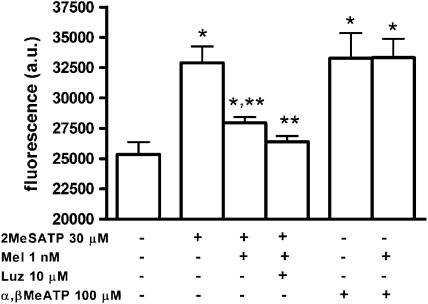
Stimulation of cultured endothelial cells for 30 min with histamine (10 μM) resulted in a significant increase of NO production that was inhibited by co-incubation with 1 nM melatonin (Figure 3a). To investigate if this effect of melatonin was mediated by a G-protein coupled MT melatonin receptor, we preincubated cells for 1 h with the nonselective MT receptor antagonist, luzindole (10 μM) and then added histamine (10 μM) and melatonin (1 nM) in the continued presence of luzindole (10 μM) for another 30 min. As shown in Figure 3a, luzindole did not prevent the melatonin effect, suggesting that it is not a receptor-mediated action. Using a protocol similar to the one just described, we found that the muscarinic agonist carbachol (100 μM) also induced NO production by endothelial cells that was sensitive to inhibition by 1 nM melatonin (Figure 3b) but was not altered by luzindole (10 μM; data not shown). However, when we used the P2 receptor agonist ATP (100 μM), we found that neither melatonin (1 nM) nor its analogue N-acetylserotonin (0.1 nM; data not shown) inhibited agonist-induced NO production (Figure 3c), even though this production was sensitive to the P2 receptor antagonist, suramin (300 μM; Figure 3c). As ATP at this concentration activates both the ionotropic P2X and the metabotropic P2Y receptors and suramin is a nonselective antagonist, we performed other experiments using α,β-methylene ATP (100 μM), an agonist selective for P2X receptors, and 2-methylthio ATP (30 μM), an agonist of P2Y receptors (Burnstock, 2006). The addition of each of these agonists for 30 min also induced NO production by endothelial cells, to an extent similar to that induced by 100 μM ATP (Figure 4). However, only the effect of 2-methylthio ATP was inhibited by 1 nM melatonin; in this case 10 μM luzindole also did not alter the effect of melatonin, as observed for histamine and carbachol and, as observed previously, for bradykinin (Tamura et al., 2006). Therefore, we can assume that the lack of effect of melatonin on ATP-mediated NO production reflects the activation of P2X receptors.
Figure 3.
Effects of melatonin on agonist-induced nitric oxide production by endothelial cells. Data are expressed as mean and s.e.m. (a) histamine 10 μM (His, n=18), melatonin 1 nM (Mel, n=18), luzindole (Luz, n=15). *P<0.01 vs basal (n=18); **P<0.01 vs histamine (one-way ANOVA), from four different cultures. (b) carbachol 100 μM (CCh, n=10), melatonin 1 nM (Mel, n=13). *P<0.01 vs basal (n=11); **P<0.01 vs carbachol (one-way ANOVA), from three different cultures. (c) ATP 100 μM (n=14), melatonin 1 nM (Mel, n=13), suramin 300 μM (Sur, n=6). *P<0.01 vs basal (n=12) and **P<0.05 vs ATP alone (one-way ANOVA), from three different cultures (except for Sur; two cultures).
Figure 4.
Investigation of the effects of melatonin on endothelial cell nitric oxide production induced by agonists of P2 receptors. Data are expressed as mean and s.e.m. 2-Methylthio ATP 30 μM (2MeSATP, n=10), 2-methylthio ATP/melatonin 1 nM (Mel, n=11), 2-methylthio ATP/melatonin/luzindole 10 μM (Luz, n=8), α,β-methylene ATP 100 μM (α,β MeATP, n=9), α,β-methylene ATP/melatonin (n=9). *P<0.01 vs basal; **P<0.01 vs 2-methylthio ATP (one-way ANOVA).
Intracellular Ca2+ measurements
Since eNOS activation depends on the Ca2+-calmodulin complex and rat endothelial cell NO production induced by agonists of G-protein coupled receptors was inhibited by 1 nM melatonin, we investigated the possibility that melatonin could be interfering with intracellular Ca2+ mobilization. For this purpose, we performed another set of experiments in a nominally Ca2+-free solution (see Methods), using confocal laser-scanning microscopy.
Because, in our system, bradykinin and ATP are agonists sensitive and insensitive to the action of melatonin, respectively, we stimulated cells with bradykinin (0.1 μM) or ATP (100 μM) to induce a maximal increase in Ca2+ (Moccia et al., 2001; Tamura et al., 2006) in the absence or presence of melatonin (1 nM), added 1 min before the agonist. The addition of melatonin or vehicle alone did not cause any alteration of resting intracellular Ca2+ levels. As illustrated in Figure 5a, we measured the fluorescence for 30 s and then added bradykinin, which induced a rapid (within 30 s) and transient increase of intracellular Ca2+ concentration (peak: 1398±188 arbitrary units, n=7), which declined completely to the pre-stimulatory level. However, the amplitude of this Ca2+ transient was reduced by melatonin (853±109 arbitrary units, n=8; P=0.022, Student's t-test). No significant alteration in the one phase exponential decay rate of this Ca2+ transient was observed (52±8 and 40±5 s in the absence or presence of melatonin, respectively). The ATP-induced increase in Ca2+ was also transient with a peak value of 1091±135 arbitrary units (n=8) but it was not altered by melatonin (894±101, n=8) (Figure 5b). In these experimental conditions, the P2X agonist α,β-methylene ATP (100 μM) did not increase fluorescence (n=20 cells), demonstrating the absence of any residual extracellular Ca2+ (Figure 5b). Considering the functional identification of P2X and P2Y receptors in these cultured endothelial cells and that only the effect of 2-methylthio ATP upon NO production was inhibited by melatonin, we repeated the protocol using this agonist. As expected, in nominally Ca2+-free solution, the activation of the P2Y receptor with 2-methylthio ATP (30 μM) induced an increase in the cytosolic concentration of Ca2+ consistent with Ca2+ mobilization. The amplitude of the response (496±53, n=17 cells) was reduced by pretreatment with melatonin (1 nM) (191±33, n=16 cells; P=0.001 Student's t-test). Furthermore, the peak value in this condition was delayed in relation to the control condition (51±5 s vs 101±15 s, respectively, P=0.001 Student's t-test) (Figure 5c).
Figure 5.
Melatonin effect upon 0.1 μM bradykinin- (a), 100 μM ATP- (b) and (c) 30 μM 2-methylthio ATP-induced increase of Ca2+ in cultured endothelial cells in nominally Ca2+-free solution. The agonists were added at 30 s. In (a) and (c), the upper and lower traces represent data obtained in the absence and presence of melatonin, respectively. In (b), the upper trace represents data obtained in the absence of melatonin, the middle trace represents data obtained in the presence of melatonin and the lower trace represents the stimulation of endothelial cells with 100 μM α,β-methylene ATP (n=20 cells). Data are expressed as mean (c; n=16–17 cells) or mean and s.e.m. (a; n=7–8 cultures, b; n=8 cultures). See Methods for details.
As both bradykinin and ATP have each been shown to mobilize Ca2+ from intracellular pools, we assessed the effects of successive addition of these agonists in a nominally Ca2+-free medium on their capacity to mobilize Ca2+ (Moccia et al., 2001). As depicted in Figure 6a, the first addition of bradykinin (0.1 μM) induced a transient increase in intracellular Ca2+. However, the subsequent application of ATP (100 μM), 6 min later, caused only a small transient increase in Ca2+. The same pattern was observed when we first added ATP and then bradykinin (Figure 6b), suggesting that part of the Ca2+ mobilized by these agonists comes from different intracellular stores.
Figure 6.
Effect of sequential stimulation of endothelial cells with Ca2+ mobilizing agonists in nominally Ca2+-free solution. (a) 0.1 μM bradykinin (upper trace) was added at 30 s and, after 6 min, 100 μM ATP was added and the increase in fluorescence recorded (lower trace). (b) 100 μM ATP (upper trace) was added at 30 s. Then 6 min later, 0.1 μM bradykinin was added and the increase in fluorescence recorded (lower trace). Data are expressed as means from 35 and 33 cells for (a) and (b), respectively, from three experiments performed with two different cultures. The s.e.m. value was omitted for clarity from upper traces.
Discussion
In the present study, we report that melatonin inhibited NO production triggered by increasing intracellular Ca2+ through stimulation of some G-protein coupled receptors, but not through the opening of receptor-operated ion channels. This effect, characterized in cultured rat endothelial cells, was also relevant for the control of arteriolar tone.
We have previously shown that melatonin acting on endothelial cells can partially inhibit the rolling and adherence of neutrophils (Lotufo et al., 2001). These effects are mediated by MT2 and MT3 receptors, respectively, since rolling was inhibited by the partial agonist of melatonin MT2 receptor, 4P-PDOT, whereas adherence was inhibited by N-acetylserotonin and 5-methoxy-carbonylamino-N-acetyl-tryptamine (5-MCA-NAT), agonists for the putative melatonin MT3 receptor. However, the pharmacological profile for inhibition of bradykinin-induced eNOS activation is quite different, since it is not modified by 4P-PDOT or 5-MCA-NAT and is not prevented by the competitive antagonist luzindole, suggesting that melatonin could modulate eNOS activity by a mechanism independent of activation of membrane receptors (Tamura et al., 2006).
As endothelial cells have been considered a putative extra-pineal source of melatonin (Kvetnoy, 1999), and such an endogenous production of melatonin could interfere with the pharmacological profile of melatonin analogues, we decided to evaluate if these cells could produce melatonin in vitro. Our experiments ruled out this possibility because we did not detect expression of the genes for the enzymes involved in the melatonin biosynthetic pathway (AA-NAT and HIOMT, Simmonneaux and Ribelayga, 2003) and the endothelial cells were not able to convert N-acetylserotonin to melatonin, the reaction catalysed by HIOMT.
The pharmacological profile of melatonin analogues suggested that the in vivo effect on leukocyte rolling, but not the in vitro effect on NO production, was mediated by MT2 receptors (Lotufo et al., 2001; Tamura et al., 2006). Data concerning the expression of MT2 melatonin receptors in endothelial cells are scarce (Masana et al., 2002). Although the MT2 melatonin receptor was reported in the intima layer of the rat tail artery (Masana et al., 2002), in our experiments, no transcription of the gene for this receptor was detected in cultured endothelial cells, in spite of the positive transcription observed in rat pineal gland. This discrepancy between in vivo and in vitro models could be related to a change in phenotype (Lang et al., 1999). In addition, the vascular endothelium, which plays an integral role in the regional specialization of vascular structures, is a highly heterogeneous tissue (Stevens et al., 2001; Frid et al., 2004; Kimura et al., 2004). Therefore, although we have used primary cultured endothelial cells to minimize any phenotypic alteration, we cannot rule out the possibility that the lack of expression of MT2 melatonin receptors is a particular feature of these cultured microvascular endothelial cells. Furthermore, both the functional and biochemical data in cultured endothelial cells indicate that melatonin acts independently of melatonin membrane receptor activation (Tamura et al., 2006; present paper). Since melatonin is a lipid-soluble molecule, it could easily enter the cell and be converted by the enzyme indoleamine 2,3-dioxygenase, expressed in endothelial cells (Beutelspacher et al., 2006), to an active metabolite (N-acetyl-5-methoxykynurenine; Ximenes et al., 2001), which was recently shown to inhibit striatal neuronal NOS (Léon et al., 2006).
Our in vivo data reinforce the importance of melatonin in regulating arteriolar vasodilation induced by NO, as melatonin inhibits both bradykinin (present work) and shear stress-induced vasodilation (Geary et al., 1998). Interestingly, a day–night variation of endothelium-dependent vasodilation induced by acetylcholine, accompanied by changes in plasma levels of NO (15.53±8.42 and 10.87±4.70 μmol l−1, at 16 and 4 h, respectively), has been described in humans (Elherik et al., 2002). Whether these vascular alterations correlate with daily variation of serum melatonin or not remains to be established.
We also evaluated whether the inhibitory effect of melatonin on NO production by endothelial cells, induced by bradykinin occurred with other known activators of endothelial NO production. We found that melatonin (1 nM) also inhibited NO production induced by carbachol and histamine, and in both cases luzindole (10 μM) did not prevent the effect of melatonin. This lack of effect of luzindole corroborated RT-PCR data in which no MT2 melatonin receptor was detected in rat endothelial cells and also discarded a MT1-mediated effect of melatonin. By contrast with agonists such as bradykinin, histamine and carbachol, ATP-induced NO production was not modified by melatonin, indicating that its inhibitory effect was not due to a general, nonspecific mechanism, such as a putative scavenger effect of melatonin upon NO (Noda et al., 1999).
Considering that ATP activates P2X ligand-gated ion channels and G-protein coupled P2Y receptors, and that several subtypes of each family are present in endothelial cells (Marrelli, 2001; Buvinic et al., 2002; Ramirez and Kunze, 2002; Volonte et al., 2006), we tested the possibility that melatonin would discriminate between them. The agonists used, 2-methylthio ATP and α,β-methylene ATP, have been shown to stimulate NO-production in endothelial cells through P2Y1 (Marrelli, 2001; Buvinic et al., 2002) and P2X4 receptors (Burnstock, 2006; Yamamoto et al., 2006), respectively. In the present study, melatonin inhibited 2-methylthio ATP-, but not α,β-methylene ATP-induced production, suggesting that only the production of NO induced by stimulation of P2Y1 receptors was blocked by melatonin.
Endothelial NOS activation depends on Ca2+-calmodulin binding, although post-translational modifications may further stimulate the enzyme (Govers and Rabelink, 2001; Venema, 2002; Bauer et al., 2003; Fleming and Busse, 2003; Kone et al., 2003). Melatonin binding to calmodulin (Benitez-King et al., 1993; Romero et al., 1998) has been proposed to be responsible for the reduction in rat cerebellum NOS activity induced by this indoleamine, in a non-saturable manner (Pozo et al., 1997). However, this was not confirmed in cultured endothelial cells, as melatonin did not mimic the effect of calmidazolium (Tamura et al., 2006).
We further explored the role of melatonin in calcium mobilization in these cells. In endothelial cells, the increase in intracellular Ca2+ is the sum of intracellular Ca2+ mobilization from thapsigargin-sensitive stores and Ca2+ influx (Oike and Ito, 1997; Cioffi et al., 2003). The disruption of Ca2+ mobilization from intracellular stores reduces bradykinin-induced cGMP production, emphasizing the relevance of the mobilization of internal stores (Gosink and Forsberg, 1993). Since bradykinin, carbachol, histamine and 2-methylthio ATP activate G-protein coupled receptors, inducing intracellular Ca2+ mobilization, and all have their effect inhibited by melatonin, we investigated if melatonin could somehow impair Ca2+ mobilization. We found that in nominally Ca2+-free solution, the maximally effective concentrations of bradykinin, ATP and 2-methylthio ATP induced a transient elevation of intracellular Ca2+. In addition, ATP, a non-selective agonist for P2Y receptors, elicited a higher signal than 2-methylthio ATP, whose effects are probably mediated by P2Y1 receptors, as described previously by Moccia et al. (2001).
Melatonin had a similar effect on bradykinin and 2-methylthio ATP-induced Ca2+ responses in reducing the peak response; however, with 2-methylthio ATP, the onset of the Ca2+ response was delayed. Melatonin did not significantly reduce the amplitude of the response to ATP. In these cells, Ca2+ pools sensitive to bradykinin and ATP do not seem to overlap completely. We observed that two sequential stimulations with bradykinin resulted in the loss of an intracellular Ca2+ increase (data not shown), whereas a second stimulus with bradykinin after ATP or vice versa, still elicited a signal. Therefore, ATP and bradykinin seem to mobilize partially overlapping pools of Ca2+.
Although bradykinin and ATP share the capacity of mobilizing intracellular Ca2+ stores, they differ in activation of eNOS in other regulatory aspects. For instance, treatment of cultured aortic endothelial cells with inhibitors of PKC abolished L-NAME-sensitive cGMP production induced by ATP, but not that induced by bradykinin (Castro et al., 1998). In addition, phosphorylation of the Ser617 and Ser635 residues of eNOS mediated by PKA in response to bradykinin and ATP in bovine aortic endothelial cells are quantitatively different (Michell et al., 2002). Finally, it was also proposed that bradykinin and ATP activate different pools of eNOS (Wagner et al., 2002). We have shown that in the presence of high extracellular Ca2+, melatonin did not alter the intracellular Ca2+ increase induced by bradykinin, thereby discarding an effect of melatonin on Ca2+ influx (Tamura et al., 2006). Therefore, we propose that the reduced Ca2+ mobilization induced by bradykinin (and 2-methylthio ATP) observed in the presence of melatonin may impair NO production in vitro and also in vivo by a not yet defined mechanism. Melatonin is known to inhibit Ca2+ mobilization from intracellular stores, albeit in another cell type (rat gonadotrophs) and without a fully defined mechanism of action (Vanecek, 1998; Dubocovich and Markowska, 2005).
Bradykinin, ATP and histamine are involved in mechanisms such as immune responses, inflammation and endothelial-mediated vasodilatation, with some of these actions being mediated by NO (Hill et al., 1997; Kunapuli and Daniel, 1998; Leeb-Lundberg et al., 2005; Burnstock, 2006). In addition, an immunomodulatory role of melatonin has been proposed (Lopes et al., 1997; Pontes et al., 2006). Thus, bearing in mind that (a) endothelial-derived NO mediates neutrophil adherence (Schaefer et al., 1998); (b) L-NAME inhibits vascular permeability induced by vascular endothelial growth factor (Murohara et al., 1998); (c) caveolin-1 knockout mice exhibit an increased vascular permeability (Schubert et al., 2002); and (d) mesenteric and cremaster microvascular hypermeability is blunted in eNOS knockout mice (Hatakeyama et al., 2006), we could propose that eNOS-derived NO is linked to the early steps of the inflammatory response and that melatonin would also regulate this function. We have already shown that low doses of melatonin inhibit paw oedema in a model of chronic inflammation in mice (Lopes et al., 1997) and vascular permeability induced by leukotriene B4 (Lotufo et al., 2006).
In conclusion, we have shown that melatonin inhibited NO production triggered by increasing intracellular Ca2+ through stimulation of some G-protein coupled receptors, but not through the opening of receptor-operated ion channels. In addition, this effect was shown to be relevant to modulation of arteriolar vasodilation in vivo and could be a mechanism underlying circadian variation of vascular tone.
Acknowledgments
The technical assistance of Débora Aparecida Moura is gratefully acknowledged. We thank Dr Eny Iochevet Segal Floh, Dr Maria Aparecida Visconti and Dr Lucile Maria Floeter-Winter who kindly allowed the use of the spectrofluorimeter, i-Cycler thermal cycler and Eppendorf Master Cycler gradient, respectively (IB, University of São Paulo).
Eduardo K Tamura is a CAPES graduate Fellow and Cláudia Lúcia Martins da Silva is a FAPESP visiting Professorial Fellow. Regina P Markus and Sandra H Farsky are CNPq Senior Fellows. FAPESP (02/02957-6) and CNPq (Brazil) supported this work.
Abbreviations
- 2-methylthio ATP
2-methylthio-adenosine triphosphate
- 4P-PDOT
4-phenyl-2-propionamidotetralin
- AA-NAT
arylalkylamine N-acetyltransferase
- DAF-FM DA
4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate
- DMEM
Dulbecco's Modified Eagle Medium
- dNTP
deoxyribonucleotide triphosphate mix
- EGTA
ethylene glycol-bis(β-aminoethyl ether) tetraacetic acid
- eNOS
endothelial nitric oxide synthase
- HIOMT
hydroxyindole-O-methyltransferase
- L-NAME
Nϖ-nitro-L-arginine methyl ester
- luzindole
N-acetyl-2-benzyltryptamine
- MT
melatonin
- OD
optical density
- PECAM-1
platelet-endothelial cell adhesion molecule 1
- RT
reverse transcriptase
Conflict of interest
The authors state no conflict of interest.
References
- Balcerczyk A, Soszynski M, Bartosz G. On the specificity of 4-amino-5-methylamino-2′,7′-difluorofluorescein as a probe for nitric oxide. Free Radic Biol Med. 2005;39:327–335. doi: 10.1016/j.freeradbiomed.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, et al. Compensatory phosphorylation and protein–protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem. 2003;278:14841–14849. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- Benitez-King G, Huerto-Delgadillo L, Anton-Tay F. Binding of 3H-melatonin to calmodulin. Life Sci. 1993;53:201–207. doi: 10.1016/0024-3205(93)90670-x. [DOI] [PubMed] [Google Scholar]
- Beutelspacher SC, Tan PH, McClure MO, Larkin DF, Lechler RI, George AJ. Expression of indoleamine 2,3-dioxygenase (IDO) by endothelial cells: implications for the control of alloresponses. Am J Transplant. 2006;6:1320–1330. doi: 10.1111/j.1600-6143.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26:412–419. doi: 10.1016/j.tips.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27:166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Buvinic S, Briones R, Huidobro-Toro P. P2Y1 and P2Y2 receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol. 2002;136:847–856. doi: 10.1038/sj.bjp.0704789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsoni S, Viswanathan M, Oliveira AM, Saavedra JM. Characterization of melatonin receptors and signal transduction system in rat arteries forming the Circle of Willis. Endocrinology. 1994;153:373–378. doi: 10.1210/endo.135.1.8013371. [DOI] [PubMed] [Google Scholar]
- Castro AF, Amorena C, Muller A, Ottaviano G, Tellez-Inon MT, Taquini AC. Extracellular ATP and bradykinin increase cGMP in vascular endothelial cells via activation of PKC. Am J Physiol. 1998;275:C113–C119. doi: 10.1152/ajpcell.1998.275.1.C113. [DOI] [PubMed] [Google Scholar]
- Cioffi DL, Wu S, Stevens T. On the endothelial cell ISOC. Cell Calcium. 2003;33:323–336. doi: 10.1016/s0143-4160(03)00046-0. [DOI] [PubMed] [Google Scholar]
- Cocks TM.Endothelium-dependent vasodilator mechanisms Pharmacology of Vascular Smooth Muscle 1996Oxford University Press: Oxford; 233–251.In: Garland CJ and Angus JA (eds) [Google Scholar]
- de Almeida-Paula LD, Costa-Lotufo LV, Silva Ferreira Z, Monteiro AE, Isoldi MC, Godinho RO, et al. Melatonin modulates rat myotube–acetylcholine receptors by inhibiting calmodulin. Eur J Pharmacol. 2005;525:24–31. doi: 10.1016/j.ejphar.2005.09.056. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- Elherik K, Khan F, McLaren M, Kennedy G, Belch JJ. Circadian variation in vascular tone and endothelial function in normal males. Clin Sci (London) 2002;102:547–552. [PubMed] [Google Scholar]
- Ferreira ZS, Cipolla-Neto J, Markus RP. Presence of P2-purinoceptors in the rat pineal gland. Br J Pharmacol. 1994;112:107–110. doi: 10.1111/j.1476-5381.1994.tb13037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes PACM, Cecon E, Markus RP, Ferreira ZS. Effect of TNF-α on the melatonin synthetic pathway in the rat pineal gland: basis for a ‘feedback' of the immune response on circadian timing. J Pineal Res. 2006;41:344–350. doi: 10.1111/j.1600-079X.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- Frid MG, Aldashev AA, Crossno JT, Jorgensen JM, Kale VA, Stenmark KR. Yin and Yang of an endothelial cell: from normal to the extreme in growth, secretion, and transdifferentiation capabilities. Paediatric Resp Rev. 2004;5:S253–S257. doi: 10.1016/s1526-0542(04)90048-6. [DOI] [PubMed] [Google Scholar]
- Geary GG, Duckles SP, Krause DN. Effect of melatonin in rat tail artery: role of K+ channels and endothelial factors. Br J Pharmacol. 1998;123:1533–1543. doi: 10.1038/sj.bjp.0701761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosink EC, Forsberg EL. Effects of ATP and bradykinin on endothelial cell Ca2+ homeostasis and formation of cGMP and prostacyclin. Am J Physiol. 1993;265:C1620–C1629. doi: 10.1152/ajpcell.1993.265.6.C1620. [DOI] [PubMed] [Google Scholar]
- Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol. 2001;280:F193–F206. doi: 10.1152/ajprenal.2001.280.2.F193. [DOI] [PubMed] [Google Scholar]
- Hatakeyama T, Pappas PJ, Hobson RW, II, Boric MP, Sessa WC, Duran WN. Endothelial nitric oxide synthase regulates microvascular hypermeability in vivo. J Physiol. 2006;574:275–281. doi: 10.1113/jphysiol.2006.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, et al. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- Kimura C, Oike M, Ohnaka K, Nose Y, Ito Y. Constitutive nitric oxide production in bovine aortic and brain microvascular endothelial cell: a comparative study. J Physiol. 2004;554:721–730. doi: 10.1113/jphysiol.2003.057059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Urano Y, Kikuchi K, Higuchi T, Hirata Y, Nagano T. Fluorescent indicators for imaging nitric oxide production. Angew Chem Int Ed Engl. 1999;38:3209–3212. doi: 10.1002/(sici)1521-3773(19991102)38:21<3209::aid-anie3209>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kone BC, Kuncewicz T, Zhang W, Yu ZY. Protein interactions with nitric oxide synthases: controlling the right time, the right place, and the right amount of nitric oxide. Am J Physiol. 2003;285:F178–F190. doi: 10.1152/ajprenal.00048.2003. [DOI] [PubMed] [Google Scholar]
- Kunapuli SP, Daniel JL. P2 receptor subtypes in the cardiovascular system. Biochem J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvetnoy IM. Extrapineal melatonin: location and role within diffuse neuroendocrine system. Histochem J. 1999;31:1–12. doi: 10.1023/a:1003431122334. [DOI] [PubMed] [Google Scholar]
- Lang D, Bell JP, Bayraktutan U, Small GR, Shah AM, Lewis MJ. Phenotypic changes in rat and guinea pig coronary microvascular endothelium after culture: loss of nitric oxide synthase activity. Cardiovasc Res. 1999;42:794–804. doi: 10.1016/s0008-6363(98)00336-8. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Léon J, Escames G, Rodriguez MI, Lopez LC, Tapias V, Entrena A, et al. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J Neurochem. 2006;98:2023–2033. doi: 10.1111/j.1471-4159.2006.04029.x. [DOI] [PubMed] [Google Scholar]
- Lopes C, de Lyra JL, Markus RP, Mariano M. Circadian rhythm in experimental granulomatous inflammation is modulated by melatonin. J Pineal Res. 1997;23:72–78. doi: 10.1111/j.1600-079x.1997.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Lotufo CMC, Lopes C, Dubocovich ML, Farsky SHP, Markus RP. Melatonin and N-acetylserotonin inhibit leukocyte rolling and adherence to rat microcirculation. Eur J Pharmacol. 2001;430:351–357. doi: 10.1016/s0014-2999(01)01369-3. [DOI] [PubMed] [Google Scholar]
- Lotufo CMC, Yamashita CE, Farsky SHP, Markus RP. Melatonin effect on endothelial cells reduces vascular permeability increase induced by leukotriene B4. Eur J Pharmacol. 2006;534:258–263. doi: 10.1016/j.ejphar.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Marrelli SP. Mechanisms of endothelial P2Y(1)- and P2Y(2)-mediated vasodilatation involve differential [Ca2+]i responses. Am J Physiol. 2001;281:H1759–H1766. doi: 10.1152/ajpheart.2001.281.4.H1759. [DOI] [PubMed] [Google Scholar]
- Masana MI, Doolen S, Ersahin C, Al-Ghoul WM, Duckles SP, Dubocovich ML, et al. MT2 melatonin receptors are present and functional in rat caudal artery. J Pharmacol Exp Ther. 2002;302:1295–1302. doi: 10.1124/jpet.302.3.1295. [DOI] [PubMed] [Google Scholar]
- Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, et al. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem. 2002;277:42344–42351. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- Moccia F, Baruffi S, Spaggiari S, Coltrini D, Berra-Romani R, Signorelli S, et al. P2Y1 and P2Y2 receptor-operated Ca2+ signals in primary cultures of cardiac microvascular endothelial cells. Microvasc Res. 2001;61:240–252. doi: 10.1006/mvre.2001.2306. [DOI] [PubMed] [Google Scholar]
- Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, et al. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]
- Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, et al. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998;427:263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- Noda Y, Mori A, Liburdy R, Packer L. Melatonin and its precursor scavenger nitric oxide. J Pineal Res. 1999;27:159–163. doi: 10.1111/j.1600-079x.1999.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Oike M, Ito Y. Dynamic regulation of intracellular Ca2+ concentration in aortic endothelial cells. Eur J Pharmacol. 1997;319:291–298. doi: 10.1016/s0014-2999(96)00846-1. [DOI] [PubMed] [Google Scholar]
- Pontes GN, Cardoso EC, Carneiro-Sampaio MM, Markus RP. Injury switches melatonin production source from endocrine (pineal) to paracrine (phagocytes) – melatonin in human colostrum and colostrum phagocytes. J Pineal Res. 2006;41:136–141. doi: 10.1111/j.1600-079X.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- Pozo D, Reiter RJ, Calvo JR, Guerrero JM. Inhibition of cerebellar nitric oxide synthase and cGMP production by melatonin via complex formation with calmodulin. J Cell Biochem. 1997;65:430–442. doi: 10.1002/(sici)1097-4644(19970601)65:3<430::aid-jcb12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Ramirez AN, Kunze DL. P2X purinergic receptor channel expression and functional in bovine aortic endothelium. Am J Physiol. 2002;282:H2106–H2116. doi: 10.1152/ajpheart.00892.2001. [DOI] [PubMed] [Google Scholar]
- Romero MP, Garcia-Perganeda A, Guerrero JM, Osuna C. Membrane-bound calmodulin in Xenopus laevis oocytes as a novel binding site for melatonin. FASEB J. 1998;12:1401–1408. doi: 10.1096/fasebj.12.13.1401. [DOI] [PubMed] [Google Scholar]
- Schaefer U, Scneider A, Rixen D, Neugebauer E. Neutrophil adhesion to histamine stimulated cultured endothelial cells is primarily mediated via activation of phospholipase C and nitric oxide synthase isozymes. Inflamm Res. 1998;47:256–264. doi: 10.1007/s000110050327. [DOI] [PubMed] [Google Scholar]
- Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, et al. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- Seltzer A, Viswanathan M, Saavedra JM. Melatonin-binding sites in brain and caudal arteries of the female rat during the estrous cycle and after estrogen administration. Endocrinology. 1992;130:1896–1902. doi: 10.1210/endo.130.4.1547717. [DOI] [PubMed] [Google Scholar]
- Simmonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 2003;55:325–395. doi: 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- Stevens T, Rosenberg R, Aird W, Quertermous T, Johnson FL, Garcia JG, et al. NHLBI workshop report: endothelial cell phenotypes in heart, lung, and blood diseases. Am J Physiol. 2001;281:C1422–C1433. doi: 10.1152/ajpcell.2001.281.5.C1422. [DOI] [PubMed] [Google Scholar]
- Tamura EK, Silva CLM, Markus RP. Melatonin inhibits endothelial nitric oxide production in vitro. J Pineal Res. 2006;41:267–274. doi: 10.1111/j.1600-079X.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- Vanecek J. Cellular mechanisms of melatonin action. Physiol Rev. 1998;78:687–721. doi: 10.1152/physrev.1998.78.3.687. [DOI] [PubMed] [Google Scholar]
- Venema RC. Post-translational mechanisms of endothelial nitric oxide synthase regulation by bradykinin. Int Immunopharmacol. 2002;2:1755–1762. doi: 10.1016/s1567-5769(02)00185-6. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Laitinen JT, Saavedra JM. Expression of melatonin receptors in arteries involved in thermoregulation. Proc Natl Acad Sci USA. 1990;87:6200–6203. doi: 10.1073/pnas.87.16.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonte C, Amadio S, D'Ambrosi N, Colpi M, Burnstock G. P2 receptor web: complexity and fine-tuning. Pharmacol Ther. 2006;112:264–280. doi: 10.1016/j.pharmthera.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Wagner S, Groschner K, Mayer B, Schmidt K. Desensitization of endothelial nitric oxide synthase by receptor agonists. Biochem J. 2002;364:863–868. doi: 10.1042/BJ20011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximenes VF, Catalani LH, Campa A. Oxidation of melatonin and tryptofan by an HRP cycle involving compound III. Biochem Biophys Res Commun. 2001;287:130–134. doi: 10.1006/bbrc.2001.5557. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12:133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- Yang Q, Scalbert E, Delagrange P, Vanhoutte PM, O'Rourke ST. Melatonin potentiates contractile responses to serotonin in isolated coronary arteries. Am J Physiol. 2001;280:H76–H82. doi: 10.1152/ajpheart.2001.280.1.H76. [DOI] [PubMed] [Google Scholar]