Abstract
Background and purpose:
Several selective oestrogen receptor modulators (SERMs) with oestrogen agonist effects in bone cells and without increased risk of breast and endometrial cancer have been developed. Here, we have investigated the effects of different types of SERMs on osteoclast differentiation, bone resorption and apoptosis in vitro.
Experimental approach:
Human peripheral blood-derived CD14+ monocytes were cultured on bovine bone slices in the presence of RANKL, M-CSF, TNF-α and dexamethasone for seven days. Also, CD14+ monocytes were co-cultured either with human SaOS-2 or MG-63 osteosarcoma cells, in the presence of parathyroid hormone. Osteoclast cultures were treated with different SERMs. TRACP+ multinucleated cells and C-terminal telopeptide of type I collagen were used as markers for osteoclast formation and bone resorption, respectively.
Key Results:
In CD14+ monocyte cultures, tamoxifen directly inhibited human osteoclast formation and bone resorption, while raloxifene and ospemifene had no inhibitory effect. In the co-cultures either with SaOS-2 or MG-63 cells, ospemifene and raloxifene as well as tamoxifen inhibited osteoclast formation in a concentration-dependent manner. The inhibitory effect was associated with an increased production of osteoprotegerin. The anti-oestrogen ICI 182 780 completely reversed the effects of these SERMs.
Conclusion and Implications:
Tamoxifen had an oestrogen receptor dependent, direct, inhibitory effect on human osteoclast differentiation and bone resorption, whereas ospemifene and raloxifene required osteoblastic cells to achieve a similar inhibition. The effects of ospemifene and raloxifene were mediated by oestrogen receptors by a mechanism involving paracrine induction of osteoprotegerin in cultures with osteoblast derived osteosarcoma cells.
Keywords: tamoxifen, ospemifene, raloxifene, osteoclast differentiation, bone resorption, osteoprotegerin
Introduction
Decrease of oestrogen in postmenopausal women results in an increased bone turnover with bone resorption exceeding bone formation. This gradually leads to a general loss of bone mass (Riggs, 1991). In aging women, decreased production of oestrogen has been associated with degenerative changes in various organ systems including the skeletal, cardiovascular and nervous system (Kanis, 1996; Halbreich, 1997; Grodstein and Stampfer, 1998). In many women, a decreasing level of oestrogen is also associated with menopausal symptoms. Oestrogen replacement therapy effectively protects against degenerative changes as well as menopausal symptoms (Marshburn and Carr, 1992). However, a long-term oestrogen therapy also increases a risk of breast and endometrial cancer (Lobo, 1995; Colditz et al., 1995). Therefore, several attempts have been taken to develop new hormone replacement therapies with beneficial effects on bone (Ward et al., 1993, Love et al., 1994), cardiovascular and nervous system (Tonetti and Jordan, 1996) in postmenopausal women, without adverse, stimulatory effects on mammary gland and uterus.
New synthetic, nonsteroidal compounds, the selective oestrogen receptor modulators (SERMs), have been recently developed and shown to possess oestrogen receptor agonistic or antagonistic selectivity in specific target tissues (Draper et al., 1996; Fournier et al., 1996; Yang et al., 1996; Shevde et al., 2000). Raloxifene is a benzothiophene SERM that specifically activates biological responses in bone tissue, without stimulation of mammary gland and uterus (Turner et al., 1988; Black et al., 1994; Fournier et al., 1996; Sato et al., 1996; Yang et al., 1996). Raloxifene binds to oestrogen receptor and it modulates gene transcription as a mixed agonist/antagonist (Compston, 2001). In bone it acts as an oestrogen agonist (Delmas et al., 1997), in mammary gland as an antagonist and in endometrium it has little effect (Delmas et al., 1997). Preclinical and clinical studies have demonstrated that raloxifene prevents bone loss and reduces a risk of fracture (De Launoit et al., 1991; Fuchs-Young et al., 1995; Evans et al., 1994, 1996). More recently, it has been shown that, similar to oestrogen, raloxifene inhibits osteoclast formation in human and mouse bone marrow cultures in vitro (Taranta et al., 2002; Ramalho et al., 2002).
Tamoxifen is a triphenylethylene SERM and it has been shown to have effects on bone by inhibiting resorption and increasing bone mineral density in postmenopausal women (Ward et al., 1993; Love et al., 1994; Powles et al., 1996) and affecting osteoclastic bone resorption in ovariectomized rats (Turner et al., 1987, 1988). Organ culture studies indicated that tamoxifen inhibited bone resorption at high concentrations (50–100 μM) (Stewart and Stern, 1986). In addition, it has been reported that tamoxifen significantly inhibited bone resorption in neonatal rat osteoclast cultures at micromolar concentrations (Arnett et al., 1996).
The recently developed ospemifene is also a triphenylethylene SERM (Qu et al., 2000). Ospemifene prevents bone loss in ovariectomized rats but its uterine effects are minor and it does not stimulate growth of human breast cancer cells either in vivo in nude mouse tumors or in vitro in cell cultures (Qu et al., 2000). However, in rat osteoclast culture ospemifene did not have any effects on osteoclast survival or bone resorption (Parikka et al., 1998). In addition, ospemifene was found to exert oestrogen-like effects in bone marrow cultures by enhancing osteoblastic differentiation with a mechanism that differs from that of raloxifene (Qu et al., 1999). The effects of ospemifene on osteoblastic differentiation could be inhibited by the pure antioestrogen ICI 182 780, suggesting an oestrogen receptor-mediated mechanism (Qu et al., 1999). In the studies of postmenopausal women, ospemifene has been reported to have bone-restoring activity similar to that of raloxifene (Komi et al., 2006).
The mechanisms by which these SERMs exert their effects on bone-resorbing human osteoclasts remain largely to be clarified. Both oestrogen receptor isoforms, oestrogen receptor-α and oestrogen receptor-β are present in osteoblasts (Eriksen et al., 1988) and osteocytes (Braidman et al., 1995). It has also been reported that oestrogen receptor-α and oestrogen receptor-β are present in osteoclasts and these results have later been confirmed in studies on human (Pensler et al., 1990; Bord et al., 2001; Braidman et al., 2001), chicken (Oursler et al., 1991) and rabbit (Mano et al., 1996). Generally, the levels of oestrogen receptors in osteoclasts are low. In human (Oreffo et al., 1999) and rat (Huang et al., 1998) studies, oestrogen receptor-α was observed in mononuclear preosteoclasts, but not in mature osteoclasts.
We have previously shown that the inhibitory effect of oestrogen on human CD14+ cell differentiation into osteoclasts is mostly mediated by osteoblastic cells and that oestrogen does not have any direct effect on osteoclast differentiation (Michael et al., 2005a). The aim of this study was to investigate the effects of the SERMs tamoxifen, raloxifene and ospemifene, on human osteoclast differentiation and bone resorption using cultures of a pure population of human peripheral blood-derived osteoclast precursors and their cocultures with human SaOS-2 or MG-63 osteoblast-derived osteosarcoma cells. We found that tamoxifen concentration-dependently inhibited both osteoclast formation and bone resorption, whereas raloxifene and ospemifene had no direct effect on CD14+ cell differentiation into active osteoclasts or resorption activity of mature osteoclasts. In the cocultures of CD14+ cells and SaOS-2 or MG-63 cells, all three SERMs concentration-dependently inhibited osteoclast formation. The oestrogen receptor antagonist ICI 182 780 completely reversed the inhibitory effects of tamoxifen in CD14+ cultures and of all SERMs in cocultures with osteoblastic cells, which suggests that both mechanisms were mediated via oestrogen receptors.
Methods
Bovine bone slices
Bone slices (7 × 7 mm, 120–140 μm thick) were cut from bovine cortical femur with a low-speed, water-cooled diamond saw (Buehler, IL, USA). Bone slices were cleaned by ultrasonification (MSE, USA) for 2 min in distilled water and then placed in 70% ethanol overnight.
Preparation of peripheral blood mononuclear cells (PBMC)
Buffy coats were obtained from blood of healthy male donors provided by the Finnish Red Cross Blood Bank (Turku, Finland). To isolate PBMC, buffy coats were diluted 1:1 (v/v) in phosphate-buffered saline (PBS), layered over Ficoll Paque Plus solution (Amersham Pharmacia Biotech, Uppsala, Sweden) and centrifuged for 30 min at 400 g. The PBMC layer was collected and washed three times with PBS and resuspended in α-minimum essential medium (α-MEM) supplemented with 9% fetal calf serum (FCS), 20 mM 4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid (HEPES), 100 IU ml−1 of pencillin and 100 μg ml−1 of streptomycin. Cells were counted using a Bürker–Türk chamber.
Preparation of purified monocytes
Immunomagnetic cell separation was performed in accordance with the manufacturer's instructions (Miltenyi Biotech, Bergisch Gladbach, Germany). PBMC were washed with magnetic activated cell sorting (MACS) buffer (PBS, pH 7.2, supplemented with 0.5% BSA and 2 mM EDTA). Clumps were removed by passing the cells through a prefilter and cells were then centrifuged at 400 g for 15 min. The cell pellet was resuspended in MACS buffer and 107 cells in 80 μl of MACS buffer were mixed with 20 μl of CD14+ antibody-coated microbeads and incubated for 15 min at 6–12°C. The cell suspension was applied to a LS-positive selection column that was previously washed with 1 ml of MACS buffer and placed in a magnetic separation unit. The column was rinsed with MACS buffer (3 × 5 ml) and unbound (CD14−) cells were discarded. The column was then removed from the magnetic separation unit and the bound cells (CD14+ monocytes) were flushed with 15 ml of MACS buffer by a syringe plunger. Finally, CD14+ monocytes were collected following centrifugation at 400 g for 10 min.
Osteoclast generation assay
A total of 1 × 105 purified CD14+ monocytes in 30 μl of cell suspension were added to a bovine bone slice placed on Parafilm and incubated for 1 h at 37°C in CO2 incubator. After the attachment period, bone slices were transferred into 48-well plates (Becton Dickinson, Franklin Lakes, NJ, USA). The cell cultures were maintained in 0.5 ml of α-MEM containing 9% FCS, 20 mM HEPES, 100 IU ml−1 penicillin, 100 μg ml−1 streptomycin, (10 ng ml−1) macrophage-colony stimulating-factor (M-CSF), (20 ng ml−1) receptor activator of nuclear factor kappa B ligand (RANKL), (10 ng ml−l) tumour necrosis factor-α (TNF-α) and dexamethasone (0.01 μM) for 7 days. SERMs at indicated concentrations (0.01–1.0 μM) were added to the medium in the beginning of cell culture. Half of the media with treatments were replaced at day 3.
Coculture assay
SaOS-2 or MG-63 cells were plated in 48-well plates (1 × 103 cells/well). Cell cultures were maintained in 1.0 ml of α-MEM containing 9% FCS, 20 mM HEPES, 100 IU ml−1 penicillin, 100 μg ml−1 streptomycin and (0.01 μM) parathyroid hormone (PTH). On the following day, 1 × 105 CD14+ monocytes/well were added and the cocultures were allowed to grow for 7 days. Half of the media were replaced once a week. SERMs (0.01–1.0 μM) were added to the cultures when monocytes were plated and when the media were changed.
Osteoclast resorption assay
Immunopurified monocytes were allowed to attach on bone slices and osteoclast formation was induced as described above. At day 7, the whole of the culture medium was replaced with fresh medium, and ospemifene, tamoxifen or raloxifene was added. Cultures were maintained for additional 3 days, cell culture media were collected and the C-terminal telopeptide of type I collagen (CTx) released from the resorbed bovine bone slices was measured to investigate osteoclastic bone resorption according to the manufacturer's instructions (Osteometer, Herlev, Denmark).
Tartrate-resistant acid phosphatase (TRACP) staining
At the end of the culture, the cells cultured either on bovine bone slices or culture plates were washed twice with PBS, fixed with 3% p-formaldehyde (PFA) (Sigma, St Louis, MO, USA) in PBS for 15 min and rewashed twice with PBS. Cells were stained for TRACP according to the instructions provided by the manufacturer (Sigma). TRACP-positive multinucleated cells (TRACP+ MNC) containing three or more nuclei were counted under the light microscope.
TRACP 5b assay
TRACP 5b in the culture medium released by osteoclasts was determined as described earlier (Alatalo et al., 2000). Briefly, rabbit anti-human TRACP antiserum was incubated in anti-rabbit immunoglobulin G-coated microtitre plates (EG & G Wallac, Turku, Finland) for 1 h. Culture medium (200 μl) was incubated in the wells for 1 h and the bound TRACP activity was detected using 8 mM 4-nitrophenyl phosphate as a substrate in 0.1 M acetate buffer for 2 h at 37°C. Adding 25 μl of 0.32 M NaOH to the wells terminated the enzyme reaction and the absorbance at 405 nm was measured with a Victor model 2 instrument (EG & G Wallac). We have already shown that the medium TRACP 5b correlates strongly (r=0.94) with the number of TRACP+ MNC formed in the culture (Alatalo et al., 2000).
Resorption pit staining
Cells were removed from bovine bone slices by soft brushing and the bone slices were rinsed extensively with PBS. Resorption pits were stained as described earlier (Selander et al., 1994). Briefly, 50 μl of peroxidase-wheat germ agglutinin (WGA)-lectin (20 μg ml−1) in PBS was pipetted onto each bone slice and incubated on Parafilm for 30 min at room temperature. Bone slices were washed twice with PBS and the resorption pits were visualized by incubating them in 3,3′ diaminobenzidine tetrahydrochloride solution (0.52 mg ml−1) for 15 min at room temperature. Finally, bovine bone slices were transferred onto glass slides, mounted with glycerol, covered with glass cover slips and observed under the light microscope.
Hoechst DNA staining
Osteoclasts were generated in eight-chamber slides (Nunc A/S, Denmark) as described above. On day 7, the whole media were replaced with fresh culture media and different concentrations of SERMs were added to the cultures and cells were allowed to grow for an additional 3 days. At the end of the culture, cells were fixed with 3% PFA for 15 min and washed twice with PBS. Hoechst stock solution in methanol was diluted in PBS (final concentration 1.25 μg ml−1) and cells were stained for 5 min in the dark. Ten microscopic fields were observed and the proportions of apoptotic osteoclasts (fragmented or shrunken nuclei) were counted under the fluorescence microscope (Leitz Aristoplan, Wetzlar, Germany) at 418 nm using × 400 magnification.
Osteoprotegerin (OPG) assay
Cell culture media from cocultures of monocytes and osteoblastic cells were collected and OPG was determined with enzyme-linked immunosorbent assay (ELISA) according to manufacturer's instructions (Biomedica, Austria). Briefly, standards, samples, positive control, assay buffer and biotin-labelled antibody were pipetted into a 96-well plate precoated with monoclonal anti-OPG antibody and mixed. After the incubation for 24 h at +4°C, the plate was washed five times with the washing buffer, streptavidin–horseradish peroxidase–conjugate was added in the wells and incubated for 1 h at room temperature. The plate was washed five times and tetramethylbenzidine was added as a substrate to develop the colour reaction. After incubation for 20 min at room temperature, stop solution was added and OPG was measured with a Victor model 2 instrument (EG & G Wallac) at 450 nm absorbance.
Statistical analysis
The results represent the mean of±s.d. of five replicates and statistical significance was analysed by Student's t-test. Statistically significant differences from control, *P<0.05, **P<0.01, ***P<0.001.
Materials
Tissue culture medium, phenol red-free α-MEM, PBS, HEPES, antibiotics and FCS were purchased from Gibco BRL (Paisley, UK). Soluble human RANKL and TNF-α were from Peprotech (London, UK). Human M-CSF was from R&D Systems (Abingdon, UK). Dexamethasone and PTH were purchased from Sigma (St Louis, MO, USA). Mouse anti-human CD14 antibody coupled to magnetic microbeads and MACS cell separation units were purchased from Miltenyi Biotech (Bergisch Gladbach, Germany). Ospemifene and tamoxifen were synthesized by Hormos Medical Ltd (Turku, Finland), whereas raloxifene was synthesized by Synthetic Chemistry Laboratory of Hormos Medical Ltd (Oulu, Finland). ICI 182 780 was purchased from Tocris Cookson (Ballwin, MO, USA).
Results
Effect of tamoxifen, ospemifene and raloxifene on human osteoclast differentiation
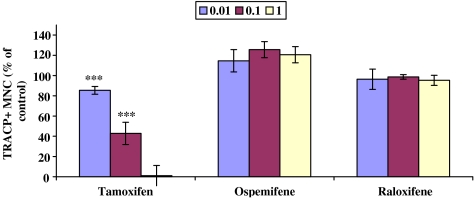
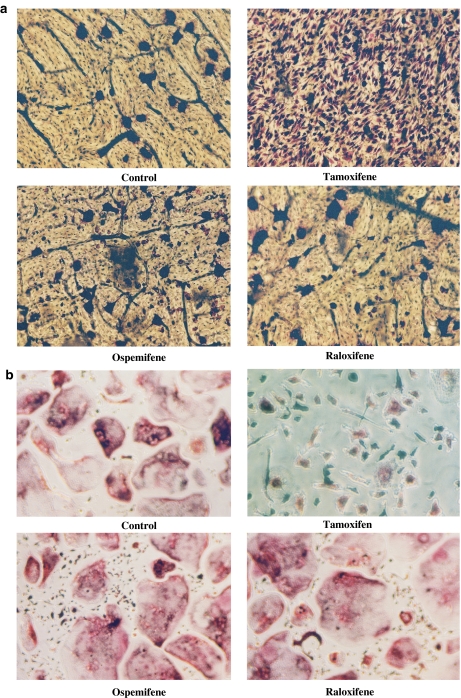
The effect of the SERMs (0.01–1 μM) on osteoclastogenesis was studied using in vitro differentiation assays. Human peripheral blood osteoclast precursors, CD14+ monocytes, were cultured on bovine bone slices in the presence of RANKL, M-CSF, TNF-α and dexamethasone for 7 days. These growth factors induced the generation of large MNC that expressed TRACP. TRACP histochemical staining was routinely used to identify osteoclasts in this study. When osteoclast precursors were cultured on bovine bone slices (Figure 1) and treated with tamoxifen, the osteoclast formation was inhibited in a dose-dependent manner as compared to the control culture (100%, nontreated culture), whereas ospemifene and raloxifene did not inhibit osteoclast formation at any given concentration. Figure 2 shows that ospemifene and raloxifene at 1 μM concentration did not inhibit TRACP+ MNC formation, whereas tamoxifen at the same concentration inhibited the formation of TRACP+ MNC without affecting the number of TRACP+ mononuclear cells.
Figure 1.
Isolated human CD14+ monocytes were cultured on bovine bone slices in the presence of RANKL, M-CSF, TNF-α and dexamethasone for 7 days and treated with or without 0.01, 0.1, 1 μM SERMs. TRACP histochemical staining was performed at the end of the culture and the number of TRACP positive-TRACP+ MNC were counted. The number of TRACP+ MNC in control cultures (=100%) was 221±18. ***P<0.001.
Figure 2.
Effect of 1 μM tamoxifen, ospemifene and raloxifene on TRACP+ MNC formation during a 7-day culture, either on bovine bone slice (a) or cell culture plate (b). Tamoxifen completely blocked the osteoclast-like cell formation without having a cytotoxic effect on other TRACP+ mononuclear cells. Microscopic magnification was × 200, except × 400 in tamoxifen-treated cultures.
Effect of tamoxifen, ospemifene and raloxifene on human osteoclastic bone resorption
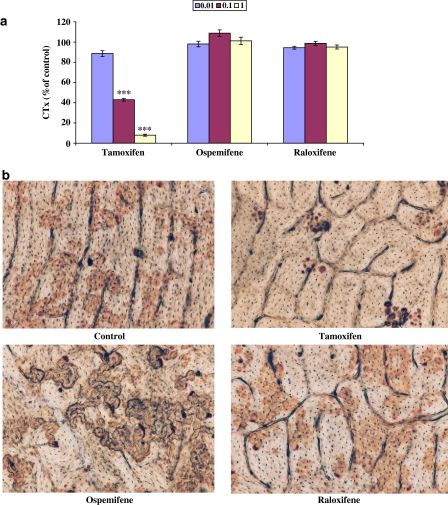
Similarly, CD14+ monocytes were cultured for 7 days on bovine bone slices to induce the differentiation of osteoclasts. The cells were then cultured for another 3 days in the presence of SERMs. CTx analysis (Figure 3a) revealed that tamoxifen (0.01–1 μM) inhibited osteoclastic bone resorption dose-dependently, compared to the control culture (100%, nontreated culture). CTx values of the cultures, where either ospemifene or raloxifene was present, were similar to the control culture. Figure 3b shows wheat WGA–lectin staining of the resorption pits on the bone slices after the resorption assay was completed. Few resorption pits were formed when osteoclast cultures were treated with tamoxifen. On the other hand, there was a significant number of resorption pits and resorption tracks when osteoclast cultures were treated with either 0.1 μM ospemifene or raloxifene showing that osteoclastic bone resorption was not inhibited by these SERMs.
Figure 3.
Effects of SERMs (0.01–1 μM) on the release of CTx by human osteoclasts when cultured on bovine bone slices. Osteoclasts were first induced to differentiate during a 7-day culture. Medium was replaced with new medium containing SERMs and cultures were continued for an additional 3 days. CTx (nM) released from the bone slices was determined as a marker for the resorption activity (a). In the control cultures (=100%), the CTx value was 32.6±2 nM. In (b), resorption pits were stained with peroxidase-conjugated WGA–lectin, after culturing osteoclasts in the presence of 0.1 μM SERMs. Microscopic magnification was × 200. ***P<0.001.
Effect of tamoxifen, ospemifene and raloxifene on osteoclast apoptosis
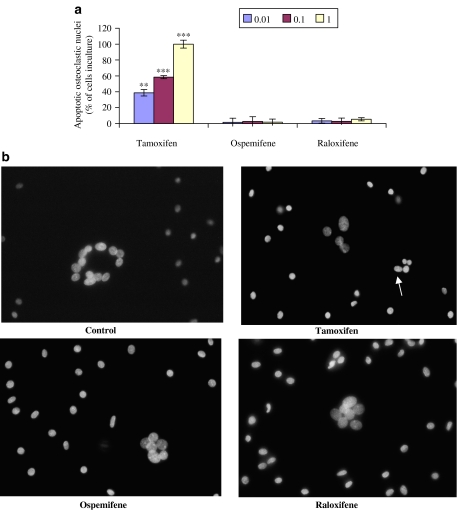
The extent of osteoclast apoptosis after treatment with different SERMs was estimated by Hoechst DNA staining (Figure 4a and b). The cultures were exposed to SERMs (0.01–1 μM) for 3 days. When human osteoclasts were treated with 1 μM tamoxifen, all the cells were apoptotic, that is, no viable osteoclasts were observed. In the cultures that were incubated with 0.01 or 0.1 μM tamoxifen, the proportion of apoptotic cells was also clearly increased, relative to the control cultures (0% apoptotic cells). In osteoclast cultures treated with ospemifene or raloxifene, the percentage of apoptotic osteoclasts was 1.6–5.4% over the whole concentration range (0.01–1 μM). However, none of these values was significantly different from those in control cultures.
Figure 4.
Hoechst DNA staining of human osteoclasts treated with or without SERMs (0.01–1 μM) for 3 days. There were no apoptotic osteoclasts in control cultures (untreated cells, 0%). The proportion of apoptotic nuclei (%) was measured in the cultures with SERMs (a). In (b), a stained culture treated with tamoxifen shows an apoptotic osteoclast (arrow). Microscopic magnification was × 400. **P<0.01; ***P<0.001.
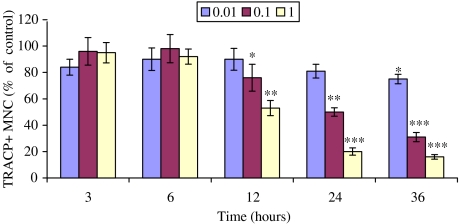
Time course of the effect of tamoxifen on human osteoclast apoptosis
As tamoxifen had a potent inhibitory effect on osteoclasts, we decided to study osteoclast apoptosis using shorter time periods (Figure 5). Human CD14+ monocytes were first induced to differentiate into osteoclasts during a 7-day culture in eight-chamber slides. Tamoxifen (0.01–1.0 μM) was then added and the cultures were continued for 3, 6, 12, 24 and 36 h. At the end of the culture, cells were fixed with 3% PFA and stained with Hoechst and TRACP staining. A significant decrease in osteoclasts was first observed at a 12 h time point in the presence of 0.1 or 1 μM tamoxifen and the numbers decreased further at 24 and 36 h. There was a 50 and 80% decrease of osteoclast numbers at a 24 h time point at concentrations of 0.1 and 1 μM, respectively. Similarly, at 36 h of culture, there was a significant decrease of osteoclast numbers, 69 and 84% compared to the control at the respective concentrations.
Figure 5.
Time course of the effect of tamoxifen on TRACP+ MNC formation in CD14+ monocyte cultures in 8-chamber slides. CD14+ monocytes were cultured either with or without tamoxifen (0.01–1 μM) for 36 h. The number of TRACP+ MNC in the control cultures was 47±7.7. *P<0.05; **P<0.01; ***P<0.001.
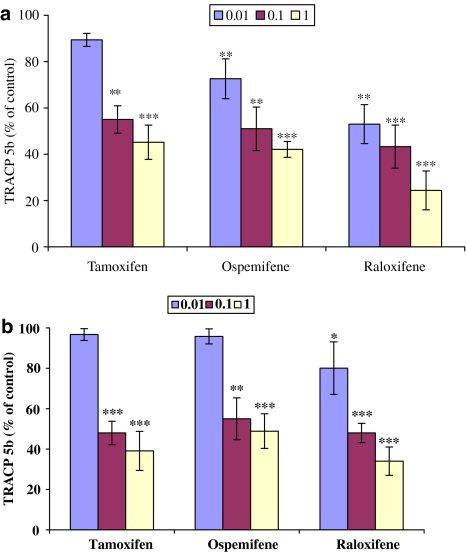
Effect of tamoxifen, ospemifene and raloxifene in the cocultures of CD14+ monocytes and SaOS-2 and MG-63 cells
We also cocultured human peripheral blood-purified CD14+ monocytes either with human SaOS-2 or MG-63 cells in the presence of PTH for 7 days (Figure 6). Formation of osteoclasts was estimated by measuring TRACP-5b released in the cell culture medium. When CD14+ monocyte and osteosarcoma cell cocultures were treated either with 0.1 or 1 μM tamoxifen, ospemifene or raloxifene, there was a marked inhibition in the formation of osteoclasts. The inhibition was approximately 50% at 0.1 μM and 63% at 1 μM compared to the control cultures. In addition, raloxifene at its lowest concentration (0.01 μM) caused a statistically significant inhibition of osteoclast formation (Figure 6a and b).
Figure 6.
Human SaOS-2 (a) or MG-63 (b) cells were cocultured for 7 days with human peripheral blood osteoclast precursors, CD14+ monocytes, in the presence of 10 nM PTH and treated with SERMs (0.01–1 μM). TRACP 5b released by osteoclasts into culture media was measured by ELISA assay (shown as units phosphatase activity l−1). The concentration of TRACP 5b in the control culture (100%) was 4.37±0.6 U l−1. *P<0.05; **P<0.01; ***P<0.001.
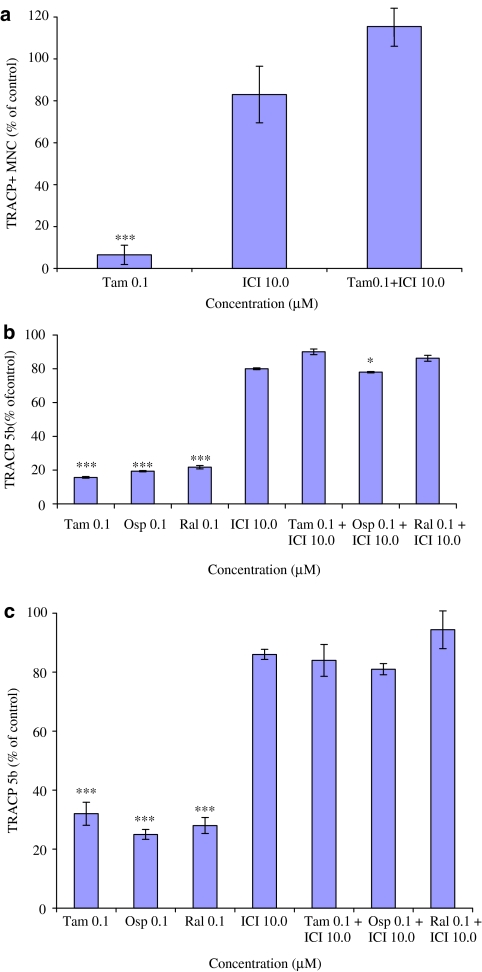
Effect of oestrogen receptor antagonist ICI 182 780 on osteoclast cultures when treated with tamoxifen, ospemifene and raloxifene
We then examined whether the inhibitory effect of SERMs was mediated via oestrogen receptor by using ICI 182 780, an oestrogen receptor antagonist. As shown in Figure 7a, in direct osteoclast culture, ICI 182 780 (in 100-fold higher concentration than tamoxifen) antagonized the inhibitory effect of tamoxifen on osteoclast formation without having any effect by itself. Similarly, when ICI 182 870 was added to CD14+and SaOS-2 cell cocultures (Figure 7b) and CD14+ and MG-63 cell cocultures (Figure 7c), it reversed the inhibitory effect of tamoxifen, ospemifene and raloxifene.
Figure 7.
Effect of 10 μM ICI 182 780 on osteoclast formation when added to CD14+ monocyte cultures with or without tamoxifen, ospemifene or raloxifene (0.1 μM). Results from CD14+ monocyte culture are shown in (a); result from CD14+ and SaOS-2 cell cocultures in (b) and results from CD14+ and MG-63 cell coculture in (c). ICI 182 780 (100-fold excess over SERM) completely antagonized the effect of tamoxifen, ospemifene or raloxifene. *P<0.05; ***P<0.001.
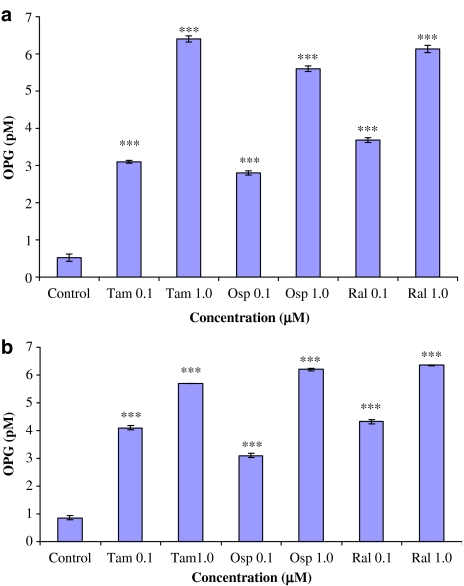
Effect of tamoxifen, ospemifene and raloxifene on production of OPG
Using a sensitive sandwich ELISA method, OPG concentrations were measured in the media collected from the cocultures of monocytes and SaOS-2 or MG-63 cells (Figure 8). In the control cultures (without tamoxifen, ospemifene or raloxifene), OPG concentration was between 0.5–0.8 pM. At 0.1 μM the SERMs caused an up to 6.4-fold stimulation of OPG production on average. At 1 μM, the stimulatory effect was approximately 12-fold in CD14+ and SaOS-2 cells cocultures, compared to the control (Figure 8a). Similarly, OPG concentrations in the medium of CD14+ and MG-63 cells cocultures grown in the presence of SERMs (0.1 and 1 μM), were increased for up to 4.7- and 7.5-fold, respectively compared to the control (Figure 8b).
Figure 8.
OPG concentrations determined in the culture media of co-cultures of SaOS-2 (a) or MG-63 (b) cells and CD14+ monocytes that were treated with ospemifene, tamoxifen or raloxifene. Note that SERMs significantly and dose-dependently stimulated OPG production in these cultures. ***P<0.001.
Discussion and conclusions
We have previously reported that high numbers of osteoclasts were produced when CD14+ monocytic precursors, from peripheral blood, were cultured in the presence of RANKL, M-CSF, TNF-α and dexamethasone with 9% FCS in α-MEM. In addition, a functional bone resorption assay was developed to estimate the bone resorbing activity of the osteoclasts formed in this culture system (Michael et al., 2005b). We preferred to use blood from male donors as it has been shown that osteoclast formation and resorption capacity is higher in male than in female (Jevon et al., 2002). Osteoclasts were, however, also formed in CD14+ monocyte cultures from female donors and the results obtained were comparable to those from the monocyte fraction from male donors.
In this study, we have examined the effects of three different SERMs, tamoxifen, ospemifene and raloxifene all of which have characteristic profiles including bone-sparing effects on differentiation of human peripheral blood-derived CD14+ monocytes into osteoclasts when cultured either on bovine bone slices or in cell culture plates. To study possible indirect effects of SERMs on osteoclast formation and function, we cocultured CD14+ cells with human SaOS-2 or MG-63 cells. Our results show that in the CD14+ monocyte differentiation assay, only tamoxifen was able to inhibit osteoclast formation whereas in cocultures all three SERMs inhibited osteoclast differentiation by a mechanism, that was associated with an increased secretion of OPG and seemed to be mediated by oestrogen receptors.
Our results are consistent with earlier studies that showed that tamoxifen inhibits osteoclastic bone resorption in ovariectomized rats (Turner et al., 1987, 1988). Tamoxifen has been reported to block bone resorption at 2–10 μM concentrations in osteoclast cultures of neonatal rats (Arnett et al., 1996) and in organ culture studies, at 50–100 μM concentrations (Stewart and Stern, 1986). In our experiments, the inhibitory effect of tamoxifen on human osteoclasts was observed at much lower concentrations (0.01–1 μM). The explanation of these differences in effective concentrations may be that we used human monocyte-derived osteoclast cultures, which may be more sensitive to tamoxifen. This was suggested by an observation that tamoxifen markedly decreased osteoclast viability without affecting mononuclear cells. The osteoclast cultures reported earlier (Arnett et al., 1996) were established from bone marrow cells, which also included osteoblasts/stromal cells, in addition to osteoclast precursors.
Tamoxifen-treated osteoclasts detached from the culture plates, their nuclei were condensed and the cells showed morphological changes associated with apoptosis. Induction of cell death may have been associated with the ionophore-like effect exerted by tamoxifen on the plasma membrane of osteoclasts (Lehenkari et al., 2003). In breast cancer cells, tamoxifen cytotoxicity has been related to nonoestrogen receptor-mediated reduction in cell membrane fluidity (Clarke et al., 1990) and to acute effects on mitochondrial function which were, however, at least partly oestrogen receptor dependent (Kallio et al., 2005). In our experiments, the ability of the anti-oestrogen ICI 182 780 to prevent inhibition of osteoclast differentiation by tamoxifen also suggests an oestrogen receptor-mediated mechanism.
In humans, tamoxifen has bone-sparing effects and the therapeutic concentration of circulating tamoxifen in humans is approximately 0.5 μM but, together with active metabolites, it can be as high as 2–3 μM (Trump et al., 1992; Peyrade et al., 1996). Our present data indicated that tamoxifen at 0.01–1 μM can be lethal to human osteoclasts cultured in a medium containing 9% FCS. It is thus possible that this effect could be involved in the bone-sparing effect of tamoxifen. An anti-resorptive skeletal profile has been firmly established for tamoxifen in postmenopausal breast cancer patients (Clemons et al., 2002; Yoneda et al., 2002). An increase in bone mineral density has been observed in them, although this has not been seen in the premenopausal patients (Powles et al., 1996). Data from breast cancer prevention trials suggest that this skeletal effect may prevent fractures as well in postmenopausal patients, although the findings were not statistically significant (Fisher et al., 1998). Experiments in rats have additionally proven that in vivo tamoxifen prevents the increase in osteoclast number and activity that is associated with the bone loss resulting from ovariectomy (Turner et al., 1987, 1988). Another SERM, toremifene, that effectively inhibits breast cancer growth and that is used in breast cancer treatment (Pagani et al., 2004) also shows some bone-sparing activity (Tiitinen et al., 2004). Its in vitro effects on osteoblastic (Lehenkari et al., 2003) and breast cancer cells (Kallio et al., 2005) are very similar to those of tamoxifen. Although not studied here, one could expect that the effects of toremifene on osteoclastogenesis to be rather similar. The major metabolites of tamoxifen and toremifene (4-hydroxy- and N-desmethylderivatives) may also contribute to bone effects in vivo but this possibility remains to be studied.
Raloxifene treatment has been demonstrated to reduce the risk of both osteoporotic vertebral fractures (Ettinger et al., 1999) and invasive breast cancer (Cummings et al., 1999) in postmenopausal women and it is becoming important in prevention of vertebral fractures in postmenopausal women. It has also been shown to inhibit bone resorption in ovariectomized rats (Evans et al., 1996). In mouse and human bone marrow cultures, raloxifene has been shown to inhibit osteoclast formation (Ramalho et al., 2002; Taranta et al., 2002) by inhibiting TNF-α action on osteoblastic cells (Taranta et al., 2002) or by upregulating transforming growth factor-β3 expression (Narayana Murthy et al., 2006). It has also been shown that raloxifene enhances OPG production and concurrently suppresses interleukin-6 (IL-6) secretion in normal human osteoblastic cells that express predominantly oestrogen receptor-α (Viereck et al., 2003). Raloxifene treatment has also been reported to downregulate the production of bone resorption-enhancing cytokines such as IL-1α, IL-1β, IL-6 and TNF-α by human osteoblastic cells and murine osteoblastic cells (Taranta et al., 2002; Cheung et al., 2003). In a clinical study of postmenopausal women, raloxifene has been shown to decrease significantly the levels of IL-6 and TNF-α in serum, whereas bone densities were increased (Gianni et al., 2004). In our experiments, raloxifene did not have inhibitory effects on the formation of osteoclasts from CD14+ cells and on the in vitro bone resorption by the osteoclasts formed. However, in the cocultures with human osteoblastic cells, raloxifene inhibited osteoclast formation. Furthermore, our results suggested that raloxifene inhibition was associated with increased production of OPG from MG-63 and SaOS-2 cells cocultured with purified monocytes. In our experiments, the level of raloxifene stimulation of OPG production was similar to that induced by estradiol, being approximately 5–10-fold higher than in control cultures (Liao et al., 2002).
Ospemifene also caused a statistically significant inhibition of osteoclast formation when CD14+ monocytes were cocultured with human osteoblastic cells. This suggests that ospemifene action was also mediated through osteoblastic cells through oestrogen receptors. Ospemifene has been found to stimulate bone formation in vitro (Qu et al., 1999) and to have bone-sparing effect and to reduce bone turnover in ovariectomized rats (Qu et al., 2000) and in postmenopausal women (Komi et al., 2004, 2006).
Our studies showed that the oestrogen receptor antagonist, ICI 182 780, completely reversed the inhibitory effect of tamoxifen in CD14+ monocyte cultures as well as that of tamoxifen, raloxifene and ospemifene in cocultures with osteoblast-derived cells. These findings suggest that the effects of these SERMs on osteoclast formation in human peripheral blood-derived osteoclast cultures are mediated by oestrogen receptors. Both MG-63 (Mahonen and Maenpaa, 1994; Vidal et al., 1999) and SaOS-2 (Sutherland et al., 1996; Vidal et al., 1999) cells express oestrogen receptors. It has also been shown that OPG production in MG-63 cells is stimulated by oestrogen and IL-1α (Vidal et al., 1998; Su et al., 2003). OPG is also produced by human osteoblastic cell line, HOB, and the human bone marrow stromal cell line, HCCL, when treated with raloxifene (Cheung et al., 2003; Viereck et al., 2003). No data on the effect of either tamoxifen or ospemifene on OPG production by osteoblastic cells have previously been available. Our results show that in cocultures, tamoxifen, raloxifene and ospemifene all have an oestrogen agonist effect on OPG production by osteoblastic cells. This is in accordance with our previous results on oestrogen effects on osteoclast differentiation (Michael et al., 2005a).
OPG has been identified as a soluble member of the TNF-receptor family (Yasuda et al., 1998), which acts as a paracrine factor within the bone microenvironment to decrease bone resorption. OPG is likely to be a major regulatory factor of bone metabolism, as it is produced by osteoblasts and stromal cells and its production is regulated by many of the major calcitropic hormones and cytokines, such as oestrogen, 1,25-dihydroxyvitamin D3, PTH, TNF-α and IL-1β (Hofbauer et al., 1998). OPG administered to normal rats increased bone density by inhibiting osteoclastogenesis and prevented bone loss following ovariectomy. Moreover, hepatic overexpression of OPG in transgenic mice induced osteopetrosis (Simonet et al., 1997) and targeted ablation of OPG led to the early onset of osteoporosis (Bucay et al., 1998; Mizuno et al., 1998).
In conclusion, our results suggest that human osteoclast formation and bone resorption is inhibited by tamoxifen both directly and indirectly through osteoblastic cells, whereas ospemifene and raloxifene seem to act indirectly only. Increased osteoblastic production of OPG was associated with the indirect SERM inhibition of osteoclastogenesis and both direct and indirect mechanisms appeared to be mediated via oestrogen receptors.
Abbreviations
- α-MEM
α-minimum essential medium
- CTx
C-terminal telopeptide of type I collagen
- FCS
fetal calf serum
- IL
interleukin
- MACS
magnetic activated cell sorting
- M-CSF
macrophage-colony-stimulating factor
- MNC
multinucleated cell
- OPG
osteoprotegerin
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- PTH
parathyroid hormone
- RANKL
receptor activator of nuclear factor kappa B ligand
- SERM
selective oestrogen receptor modulator
- TNF
tumour necrosis factor
- TRACP
tartrate-resistant acid phosphatase
- WGA
wheat germ agglutinin
Conflict of interest
The authors state no conflict of interest.
References
- Alatalo SL, Halleen JM, Hentunen TA, Monkkonen J, Vaananen HK. Rapid screening method for osteoclast differentiation in vitro that measures tartrate-resistant acid phosphatase 5b activity secreted into the culture medium. Clin Chem. 2000;46:1751–1754. [PubMed] [Google Scholar]
- Arnett TR, Lindsay R, Kilb JM, Moonga BS, Spowage M, Dempster DW. Selective toxic effects of tamoxifen on osteoclasts, comparison with the effects of oestrogen. J Endocrinol. 1996;149:503–508. doi: 10.1677/joe.0.1490503. [DOI] [PubMed] [Google Scholar]
- Black LJ, Sato M, Rowley ER, Magee DE, Bekele A, Williams DC, et al. Roloxifene ( LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest. 1994;207:19–37. doi: 10.1172/JCI116985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bord S, Horner A, Beavan S, Compston J. Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab. 2001;86:2309–2314. doi: 10.1210/jcem.86.5.7513. [DOI] [PubMed] [Google Scholar]
- Braidman IP, Davenport LK, Carter DH, Selby PL, Mawer EB, Freemont AJ. Preliminary in situ identification of estrogen target cells in bone. J Bone Miner Res. 1995;10:74–80. doi: 10.1002/jbmr.5650100112. [DOI] [PubMed] [Google Scholar]
- Braidman IP, Hainey L, Batra G, Selby PL, Saunders PT, Hoyland JA. Localization of estrogen receptor beta protein expression in adult human bone. J Bone Miner Res. 2001;16:214–220. doi: 10.1359/jbmr.2001.16.2.214. [DOI] [PubMed] [Google Scholar]
- Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung J, Mak YT, Papaioannou S, Evans BA, Fogelman I, Hampson G. Interleukin-6 (IL-6), IL-1, receptor activator of nuclear factor kappaB ligand (RANKL) and osteoprotegerin production by human osteoblastic cells, comparison of the effects of 17-beta oestradiol and raloxifene. J Endocrinol. 2003;177:423–433. doi: 10.1677/joe.0.1770423. [DOI] [PubMed] [Google Scholar]
- Clarke R, van der Berg HW, Murphy RF. Reduction of the membrane fluidity of human breast cancer cells by tamoxifen and 17beta-estradiol. J Natl Cancer Inst. 1990;82:1702–1705. doi: 10.1093/jnci/82.21.1702. [DOI] [PubMed] [Google Scholar]
- Clemons M, Danson S, Howel A. Tamoxifen (Nolvadex), a review. Cancer Treat Rev. 2002;28:165–180. doi: 10.1016/s0305-7372(02)00036-1. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, Stampfer MJ, et al. The use of estrogen and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- Compston JE. Sex steroids and bone. Physiol Rev. 2001;81:419–447. doi: 10.1152/physrev.2001.81.1.419. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women, results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- De Launoit Y, Veilleux R, Dufour M, Simard J, Labrie F. Characteristics of the biphasic action of androgens and of the potent antiproliferative effects of the new pure antiestrogen EM-139 on cell cycle kinetic parameters in LNCaP human prostatic cancer cells. Cancer Res. 1991;51:5165–5170. [PubMed] [Google Scholar]
- Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, et al. Effect of raloxifene on bone mineral density, serum cholesterol concerntrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- Draper MW, Flowers DE, Huster WJ, Neild JA, Harper KD, Arnaud C. A controlled trial of raloxifene ( LY139481) HCL, impact on bone turnover and serum lipid profile in healthy postmenopausal women. J Bone Miner Res. 1996;11:835–842. doi: 10.1002/jbmr.5650110615. [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelsberg TC, et al. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988;241:84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene, results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- Evans G, Bryant HU, Magee D, Sato M, Turner RT. The effects of raloxifene on tibia histomorphometry in ovariectomized rats. Endocrinology. 1994;134:2283–2288. doi: 10.1210/endo.134.5.8156931. [DOI] [PubMed] [Google Scholar]
- Evans GL, Bryant HU, Magee DE, Turner RT. Raloxifene inhibits bone turnover and prevents further cancellous bone loss in adult overectomized rats with estabilished osteopenia. Endocrinology. 1996;137:4139–4144. doi: 10.1210/endo.137.10.8828469. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer, report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Int. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Fournier B, Haring S, Kaye AM, Somjen D. Stimulation of creatine kinase specific activity in human osteoblast and endometrial cells by estrogens and anti-estrogens and its modulation by calciotropic hormones. J Endocrinol. 1996;150:275–285. doi: 10.1677/joe.0.1500275. [DOI] [PubMed] [Google Scholar]
- Fuchs-Young R, Glasebrook AL, Short LL, Draper MW, Ripply MK, Cole HW, et al. Raloxifene is a tissue selective agonist/antagonist that functions through the estrogen receptor. Ann NY Acad Sci. 1995;761:355–360. doi: 10.1111/j.1749-6632.1995.tb31392.x. [DOI] [PubMed] [Google Scholar]
- Gianni W, Ricci A, Gazzaniga P, Brama M, Pietropaolo M, Votano S, et al. Raloxifene modulated interleukin-6 and tumor necrosis factor-α synthesis in vivo: results from a pilot clinical study. J Clin Endocrinol Metab. 2004;89:6097–6099. doi: 10.1210/jc.2004-0795. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Stampfer MJ. Estrogen for women at varying risk of coronary disease. Maturitas. 1998;30:19–26. doi: 10.1016/s0378-5122(98)00055-3. [DOI] [PubMed] [Google Scholar]
- Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48:16–19. doi: 10.1212/wnl.48.5_suppl_7.16s. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Osteoprotegrin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein and cytokines. Biochem Biophys Res Commun. 1998;250:776–781. doi: 10.1006/bbrc.1998.9394. [DOI] [PubMed] [Google Scholar]
- Huang WH, Lau AT, Daniels LL, Fujii H, Seydel U, Wood DJ, et al. Detection of estrogen receptor-α, carbonic anhydrase II and tartrate-resistant acid phosphatase mRNAs in putative mononuclear osteoclast precursor cells of neonatal rats by fluorescence in situ hybridization. J Mol Endocrinol. 1998;20:211–219. doi: 10.1677/jme.0.0200211. [DOI] [PubMed] [Google Scholar]
- Jevon M, Sabokbar A, Fujikawa Y, Hirayama T, Neale SD, Wass J, et al. Gender- and age-related differences in osteoclast formation from circulating precursors. J Endocrinol. 2002;172:673–681. doi: 10.1677/joe.0.1720673. [DOI] [PubMed] [Google Scholar]
- Kallio A, Zheng A, Heiskanen K, Harkonen P. Role of mitochondria in tamoxifen-induced death of MCF-7 breast cancer cells. Apoptosis. 2005;10:1395–1410. doi: 10.1007/s10495-005-2137-z. [DOI] [PubMed] [Google Scholar]
- Kanis JA. Estrogens, the menopause, and osteoporosis. Bone. 1996;19:185–190. doi: 10.1016/s8756-3282(96)90163-5. [DOI] [PubMed] [Google Scholar]
- Komi J, Heikkinen J, Rutanen EM, Halonen K, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on biochemical markers of bone turnover in healthy postmenopausal women. Gynecol Endocrinol. 2004;18:152–158. doi: 10.1080/09513590410001672197. [DOI] [PubMed] [Google Scholar]
- Komi K, Lankinen KS, Degegorio M, Heikkinen J, Saarakoski S, Tuppurainen M, et al. Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. J Bone Miner Metab. 2006;24:314–318. doi: 10.1007/s00774-006-0689-9. [DOI] [PubMed] [Google Scholar]
- Lehenkari P, Parikka V, Rautila TJ, Weckstrom M, Dahllund J, Harkonen PL, et al. The effects of tamoxifen and toremifene on bone cells involve changes in plasma membrane ion conductance. J Bone Miner Res. 2003;18:473–481. doi: 10.1359/jbmr.2003.18.3.473. [DOI] [PubMed] [Google Scholar]
- Liao EY, Luo XH, Su X. Comparison of the effects of 17beta-E2 and progesterone on the expression of osteoprotegerin in normal human osteoblast-like cells. J Endocrinol Invest. 2002;25:785–790. doi: 10.1007/BF03345513. [DOI] [PubMed] [Google Scholar]
- Lobo RA. Benefits and risks of estrogen replacement therapy. Am J Obstet Gynecol. 1995;173:982–990. doi: 10.1016/0002-9378(95)90247-3. [DOI] [PubMed] [Google Scholar]
- Love RR, Barden HS, Mazess RB, Epstein S, Chappell RJ. Effect of tamoxifen on lumbar spine bone mineral density in postmenopausal women after 5 years. Arch Intern Med. 1994;28:2585–2588. [PubMed] [Google Scholar]
- Mahonen A, Maenpaa PH. Steroid hormone modulation of vitamin D receptor levels in human MG-63 osteosarcoma cells. Biochem Biophys Res Commun. 1994;205:1179–1186. doi: 10.1006/bbrc.1994.2790. [DOI] [PubMed] [Google Scholar]
- Mano H, Yuasa T, Kameda T, Miyazawa K, Nakamaru Y, Shiokawa M, et al. Mammalian mature osteoclasts as estrogen target cells. Biochem Biophys Res Commun. 1996;223:637–642. doi: 10.1006/bbrc.1996.0947. [DOI] [PubMed] [Google Scholar]
- Marshburn PB, Carr BR. Hormone replacement therapy, protection against the consequences of menopause. Postgrad Med J. 1992;92:145–159. doi: 10.1080/00325481.1992.11701471. [DOI] [PubMed] [Google Scholar]
- Michael H, Harkonen PL, Vaananen HK, Hentunen TA. Estrogen and testosterone inhibit osteoclastogenesis through different cellular pathways. J Bone Miner Res. 2005a;20:2224–2232. doi: 10.1359/JBMR.050803. [DOI] [PubMed] [Google Scholar]
- Michael H, Nyman JK, Vaaraniemi J, Vaananen HK, Hentunen TA. Characterization of circulating human osteoclast progenitors, development of in vitro resorption assay. Calcif Tissue Int. 2005b;76:222–230. doi: 10.1007/s00223-004-0123-z. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610–615. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- Narayana Murthy PS, Sengupta S, Sharma S, Singh MM. Effects of ormeloxifene on ovarioectomy-induced bone resorption, osteoclast differentiation and apoptosis and TGF-beta-3 expression. J Steroid Biochem Mol Biol. 2006;100:117–128. doi: 10.1016/j.jsbmb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Oreffo RO, Kusec V, Vidi AS, Flangan AM, Grano M, Zambonin-Zallone A, et al. Expression of estrogen receptor-α in cells of the osteoclastic lineage. Histochem Cell Biol. 1999;111:125–133. doi: 10.1007/s004180050342. [DOI] [PubMed] [Google Scholar]
- Oursler MJ, Osdoby P, Pyfferoen J, Riggs BL, Spelsberg TC. Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci USA. 1991;88:6613–6617. doi: 10.1073/pnas.88.15.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani O, Gelber S, Price K, Zahrieh D, Gelber R, Simonici E, et al. Toremifene and tamoxifen are equally effective for early-stage breast cancer: first results of International Breast Cancer Study Group Trials 12–93 and 14–93. Ann Oncol. 2004;15:1749–1759. doi: 10.1093/annonc/mdh463. [DOI] [PubMed] [Google Scholar]
- Parikka V, Lehenkari PP, Harkonen PK, Vaananen HK. The effect of two selective estrogens raloxifene and FC1271a on osteoclast survival and resorption in vitro. Bone. 1998;23 Suppl:S552. [Google Scholar]
- Pensler JM, Langman CB, Radosevich JA, Maminta ML, Mangkornkanok M, Higbee R, et al. Sex steroid hormone receptors in normal and dysplastic bone disorders in children. J Bone Miner Res. 1990;5:493–498. doi: 10.1002/jbmr.5650050511. [DOI] [PubMed] [Google Scholar]
- Peyrade F, Frenay M, Etienne MC, Ruch F, Guillemare C, Francois E, et al. Age-related difference in tamoxifen disposition. Clin Pharmacol Ther. 1996;59:401–410. doi: 10.1016/S0009-9236(96)90108-3. [DOI] [PubMed] [Google Scholar]
- Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- Qu Q, Zheng H, Dahllund J, Laine A, Cockcroft N, Peng Z, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809–820. doi: 10.1210/endo.141.2.7342. [DOI] [PubMed] [Google Scholar]
- Qu Q, Harkonen PL, Vaananen HK. Comparative effects of estrogen and antiestrogens on differentiation of osteoblasts in mouse bone marrow culture. J Cell Biochem. 1999;73:500–507. [PubMed] [Google Scholar]
- Ramalho AC, Couttet P, Baudoin C, Morieux C, Graulet AM, De Vernejoul MC, et al. Estradiol and raloxifene decrease the formation of multinucleate cells in human bone marrow cultures. Eur Cytokine Netw. 2002;13:39–45. [PubMed] [Google Scholar]
- Riggs BL. Overview of osteoporosis. West J Med. 1991;154:63–77. [PMC free article] [PubMed] [Google Scholar]
- Sato M, Rippy MK, Bryant HU. Raloxifene, tamoxifene, nofoxidine or estrogen effects on reproductive and non-reproductive tissue in overiectomized rats. FASEB. 1996;10:905–912. doi: 10.1096/fasebj.10.8.8666168. [DOI] [PubMed] [Google Scholar]
- Selander K, Lehenkari P, Vaananen HK. The effects of bisphosphonates on the resorption cycle of isolated osteoclasts. Calcif Tissue Int. 1994;55:368–375. doi: 10.1007/BF00299317. [DOI] [PubMed] [Google Scholar]
- Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-jun expression. Proc Natl Acad Sci USA. 2000;97:7829–7834. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin a novel secreted protein involved in regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Stewart PJ, Stern PH. Effects of the antiestrogens tamoxifen tamoxifen and clomiphene on bone resorption in vitro. Endrocrinology. 1986;118:125–131. doi: 10.1210/endo-118-1-125. [DOI] [PubMed] [Google Scholar]
- Su X, Liao EY, Peng J, Wu XP. The effects of 17 beta-estradiol on the expression of osteoprotegerin, the ligand of osteoprotegerin and related cytokines in osteosarcoma MG63 cells. Zhonghua Nei Ke Za Zhi. 2003;42:800–803. [PubMed] [Google Scholar]
- Sutherland MK, Hui DU, Rao LG, Wylie JN, Murray TM. Immunohistochemical localization of the estrogen receptor in human osteoblastic SaOS-2 cells, association of receptor levels with alkaline phosphatase activity. Bone. 1996;18:361–369. doi: 10.1016/8756-3282(96)00016-6. [DOI] [PubMed] [Google Scholar]
- Taranta A, Brama M, Teti A, De Luca V, Scandurra R, Spera G, et al. The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro. Bone. 2002;30:368–376. doi: 10.1016/s8756-3282(01)00685-8. [DOI] [PubMed] [Google Scholar]
- Tiitinen A, Nikander E, Hietanen P, Metsa-Heikkila M, Ylikorkela O. Changes in bone mineral density during and after 3 years' use of tamoxifen or toremifene. Maturitas. 2004;48:321–327. doi: 10.1016/j.maturitas.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Tonetti DA, Jordan VC. Targeted anti-estrogens to treat and prevent diseases in women. Mol Med Today. 1996;5:218–223. doi: 10.1016/1357-4310(96)88775-2. [DOI] [PubMed] [Google Scholar]
- Trump DL, Smith DC, Ellis PG, Rogers MP, Schold SC, Winer EP, et al. High-dose oral tamoxifen, a potential multidrug-resistance-reversal agent, phase I trial in combination with vinblastine. J Natl Cancer Inst. 1992;84:1811–1816. doi: 10.1093/jnci/84.23.1811. [DOI] [PubMed] [Google Scholar]
- Turner RT, Wakley GK, Hannon KS, Bell NH. Tamoxifen prevents the skeletal effects of ovarian hormone deficiency in rats. J Bone Miner Res. 1987;2:449–456. doi: 10.1002/jbmr.5650020513. [DOI] [PubMed] [Google Scholar]
- Turner RT, Wakley GK, Hannon KS, Bell NH. Tamoxifen inhibits osteoclast-mediated resorption of trabecular bone in ovarian hormone-deficient rats. Endocrinology. 1988;122:1146–1150. doi: 10.1210/endo-122-3-1146. [DOI] [PubMed] [Google Scholar]
- Vidal O, Kindblom LG, Ohlsson C. Expression and localization of estrogen receptor-beta in murine and human bone. J Bone Miner Res. 1999;14:923–929. doi: 10.1359/jbmr.1999.14.6.923. [DOI] [PubMed] [Google Scholar]
- Vidal ON, Sjogren K, Eriksson BI, Ljunggren O, Ohlsson C. Osteoprotegerin mRNA is increased by interleukin-1 alpha in the human osteosarcoma cell line MG-63 and in human osteoblast-like cells. Biochem Biophys Res Commun. 1998;248:696–700. doi: 10.1006/bbrc.1998.9035. [DOI] [PubMed] [Google Scholar]
- Viereck V, Grundker C, Blaschke S, Niederkleine B, Siggelkow H, Frosch KH, et al. Raloxifene concurrently stimulates osteoprotegerin and inhibits interleukin-6 production by human trabecular osteoblasts. J Clin Endocrinol Metab. 2003;88:4206–4213. doi: 10.1210/jc.2002-021877. [DOI] [PubMed] [Google Scholar]
- Ward RL, Morgan G, Dalley D, Kelley PJ. Tamoxifen reduces bone turnover and prevents lumbar spine and proximal femoral bone loss in early postmenopausal women. Bone Miner. 1993;22:87–94. doi: 10.1016/s0169-6009(08)80220-6. [DOI] [PubMed] [Google Scholar]
- Yang NN, Venugopalan M, Hardikar S, Glasebrook A. Identification of anti estrogen response element activated by metabolities of estradiol and raloxifene. Science. 1996;273:1222–1225. doi: 10.1126/science.273.5279.1222. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANC/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K, Tanji Y, Ikeda N, Miyoshi Y, Taguchi T, Tamaki Y, et al. Influence of adjuvant tamoxifen treatment on bone mineral density and bone turnover markers in postmenopausal breast cancer patients in Japan. Cancer Lett. 2002;186:223–230. doi: 10.1016/s0304-3835(02)00345-2. [DOI] [PubMed] [Google Scholar]