Abstract
During the 1980s, the free radical, nitric oxide (NO), was discovered to be a crucial signalling molecule, with wide-ranging functions in the cardiovascular, nervous and immune systems. Aside from providing a credible explanation for the actions of organic nitrates and sodium nitroprusside that have long been used in the treatment of angina and hypertensive crises respectively, the discovery generated great hopes for new NO-based treatments for a wide variety of ailments. Decades later, however, we are still awaiting novel licensed agents in this arena, despite an enormous research effort to this end. This review explores some of the most promising recent advances in NO donor drug development and addresses the challenges associated with NO as a therapeutic agent.
Keywords: nitric oxide, nitric oxide donor drugs, S-nitrosothiol, diazeniumdiolate, NONOate, furoxan, nitroaspirin, organic nitrate
Introduction
In 1992, the free radical, nitric oxide (formula •N=O, abbreviated to NO), was awarded the curious accolade of ‘molecule of the year' (Culotta and Koshland, 1992) and the extensive body of research already built around this small molecule continues to increase unabated, with ∼13 000 papers focused on the topic in the past 5 years alone. Despite its structural simplicity, NO has a complex chemistry, endowing the free radical with wide and varied biological actions. NO is synthesized by a number of cell types, where it acts as a highly regulated autocrine and paracrine signalling molecule. Importantly, its sphere of influence is likely to only extend to ∼100 μm of its origin on account of its reactive nature. This property of NO has important consequences: first, it means that NO must be rapidly synthesized on demand in response to stimuli and second, it is truly a local mediator that does not require complex metabolism for clearance; it is simply diluted and oxidized to nitrite and nitrate as it diffuses away from its source. This property is both the greatest weakness and strength of NO in relation to its use as a therapeutic agent, as will be discussed later. It is worth noting, however, that nitrite (Gladwin et al., 2006) and/or some of the possible sinks for NO (haemoglobin; Singel and Stamler, 2005, albumin; Crane et al., 2002) are not necessarily inactive by-products but might themselves be complex NO ‘donors'. This aspect of NO biology is highly complex and contentious (Hobbs et al., 2002) and is beyond the remit of this review.
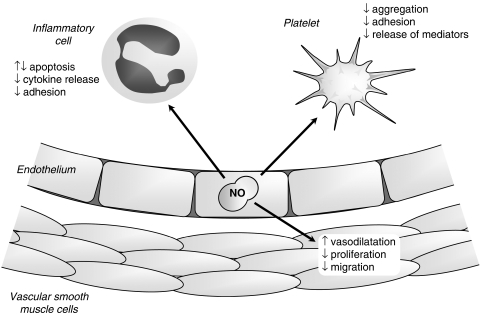
The most studied actions of NO are in the cardiovascular system (Figure 1), where it is continuously produced by the endothelial cells that line the lumen of blood vessels. Here, L-arginine is converted into NO by the endothelial isoform of the enzyme, NO synthase (eNOS), in response to mechanical and chemical stimuli that act by mobilizing intracellular calcium. NO diffuses in three dimensions away from the cell of origin, passing easily through membranes of neighbouring cells, where it can bring about a range of physiological effects. In vascular smooth muscle cells (VSMCs), it acts almost exclusively (Miller et al., 2004) via the enzyme soluble guanylate cyclase (sGC) to stimulate the generation of cyclic guanosine-3′,5′-monophosphate (cGMP) and elicit vasodilatation via cGMP-dependent protein kinases. NO is also recognized to inhibit VSMC proliferation by a cGMP-dependent mechanism (Jeremy et al., 1999). Elsewhere, NO also has an impact on circulating platelets, where both cGMP-dependent and -independent (Crane et al., 2005) mechanisms in response to endothelium and platelet-derived NO play a part in a powerful inhibitory effect on aggregation and adhesion. Similarly, endothelium-derived NO is a powerful inhibitor of inflammatory cell activation and, most notably, is recognized to be an important inhibitor of monocyte activity (Bath, 1993). Interestingly, the relative role of NO compared to other endothelium-derived vasodilators (prostacyclin, endothelium-derived hyperpolarizing factor; EDHF) is not uniform across the vascular system: NO generally predominates in large conduits, whereas EDHF is apparently dominant in resistance vessels. This localization of NO to conduits that have little impact on blood pressure determination might suggest that the primary role of NO lies its antiatherothrombotic properties rather than its vasodilator effects, a suggestion that is supported by the evident impact of endothelial dysfunction in conduit arteries on atherogenesis.
Figure 1.
The multifaceted action of NO in the cardiovascular system.
It is important to recognize that the concentrations of NO required to mediate the primarily protective effects described above are extremely low (picomolar to nanomolar). An important feature of NO is that its properties and cellular targets at higher concentrations are profoundly different, particularly under conditions of oxidative stress, where it rapidly reacts with superoxide to form peroxynitirite (ONOO−). Under these circumstances, NO is highly cytotoxic, a feature that is exploited by inflammatory cells in response to invading pathogens by expressing an inducible form of NOS (iNOS) in concert with activation of NAD(P)H oxidase to cogenerate NO and superoxide, forming highly cytotoxic and cytostatic ONOO−. At high concentrations, additional chemical reactions become relevant, especially those with molecular oxygen, generating nitrosating species capable of regulating protein and cell function (Gow et al., 2004). Of particular note is the inhibitory impact of NO, together with endogenous S-nitrosothiols and ONOO− on cellular respiration through interaction with complexes in the respiratory chain (Beltran et al., 2000; Brown and Borutaite, 2004). High concentrations of NO and related species also mediate apoptosis in inflammatory cells (Taylor et al., 2003).
Endothelial dysfunction
Endothelial dysfunction is implicated in the development of a wide range of cardiovascular risk factors and conditions including atherosclerosis (Ludmer et al., 1986), heart failure (Katz et al., 1992), diabetes (Calver et al., 1992b), hypertension (Calver et al., 1992a), cigarette smoking (Newby et al., 1999), hypercholesterolaemia (Drexler and Zeiher, 1991). Depression of the NO:sGC pathway is a key feature of endothelial dysfunction, occurring at a number of levels, including downregulation of eNOS expression and activity, uncoupling of NOS, scavenging of NO by oxygen-centred free radicals and decreased sensitivity of VSMCs to vasodilators, together with NO-independent alterations (Feletou and Vanhoutte, 2006). Loss of endogenous NO activity has a number of detrimental actions, most notably, vasoconstriction, increased smooth muscle cell proliferation, as well as increased activity and adherence of platelets and inflammatory cells at sites of endothelial damage. As the endothelium deteriorates, blood flow is disturbed and vessels become occluded by atheromatous plaque, or their associated thrombosis or emboli, ultimately leading to infarction of downstream organs, resulting in myocardial infarction, stroke and peripheral ischaemia.
At first sight, delivery of exogenous NO is an attractive therapeutic option, particularly with a view to slowing progression of atherosclerosis and reducing the risk of thrombosis, on account of its multifaceted action. NO from existing organic nitrates effectively alleviates symptoms of angina through improved blood flow to the ischaemic region of the heart via dilatation of diseased vessels and collateral coronary arteries. Systemic dilatation of veins and resistance arteries also reduces both pre- and afterload, reducing the cardiac workload and oxygen consumption and limiting the pain associated with hypoxia. In addition, however, NO would be expected to inhibit VSMC proliferation and migration, limiting the development of the complex plaque, and inhibit platelet activation, aggregation and adhesion to areas of damage, reducing the extent of thrombosis. Furthermore, NO would be expected to inhibit inflammatory cell activation, preventing infiltration into the plaque and also the propagation of proinflammatory signals. Unfortunately, however, the latter benefits do not appear to be realized with organic nitrates and these drugs remain effective only as symptomatic treatments, rather than agents that might slow disease progression and improve outcome (see below). Whilst the multifaceted impact of NO should, at least in theory, constitute a benefit in this setting, it might equally be argued that the enormous variety of effects of NO in different tissues and systems might be a considerable limitation to systemic NO delivery, with unwanted side effects outside the target tissue.
NO donor drugs
NO gas is notoriously difficult to handle on account of the problems associated with complete exclusion of oxygen to prevent oxidation to nitrogen dioxide. Nevertheless, the gas itself can be used therapeutically, particularly in pulmonary hypertension (Griffiths and Evans, 2005) and in neonates (Greenough, 2000), where it is delivered to the lungs via inhalation. This specific use apart, however, we are reliant on molecular carriers of NO (NO donor drugs) to stabilize the radical until such time as its release is required. Given the enormous potential of NO in cardiovascular medicine, it is somewhat astonishing that only two types of NO donor drug are currently used clinically and no new NO donors have reached the market since the discovery of NO as a crucial cardiovascular mediator in the 1980s. Recently, there have been a plethora of reviews on NO donor drugs, providing detailed descriptions of the different classes and neatly summarizing decades of research (Megson, 2000; Yamamoto and Bing, 2000; Burgaud et al., 2002; Ignarro et al., 2002; Megson and Webb, 2002; Napoli and Ignarro, 2003). This review will therefore focus on recent advances in NO donor drug development within the past 5–10 years, with special attention to diazeniumdiolates, S-nitrosothiols, NO donor hybrid drugs and NO-generating materials that show particular promise. Although the primary focus of the review is the prevention or treatment of cardiovascular conditions, it also highlights novel drug design with other goals in mind.
NO donors in current clinical use
Before considering novel NO donor drugs, it is necessary to briefly comment on existing drugs because there are several new studies that have expanded our understanding of the compounds and might alter clinical practice.
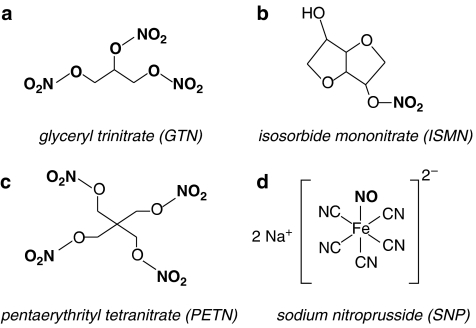
The organic nitrates are the most commonly used NO donor drugs. Glyceryl trinitrate (GTN; also known as nitroglycerin; Figure 2a) is the best-studied nitrate, used mainly in acute relief of pain associated with angina, whereas other slower release preparations, such as isosorbide mononitrate (ISMN; Figure 2b), are used for the treatment of chronic angina. GTN ointments are also routinely used for the treatment of anal fissure (Fenton et al., 2006), transdermal patches in heart failure and chronic angina, whereas nebulized GTN may have benefits in certain subgroups with pulmonary hypertension (Yurtseven et al., 2003; Goyal et al., 2006). GTN contains three nitro-oxy ester (from now on referred to as nitrate) groups, but releases only one molar equivalent of NO from the terminal position after bioactivation (Bennett et al., 1989). However, the metabolism of GTN to NO has strict requirements beyond that of simple chemical reduction and it is now almost universally agreed that specific enzymes mediate this process in vivo (Thatcher et al., 2004), although the requirement for NO release at all was recently challenged in an interesting paper (Kleschyov et al., 2003). The identity of the nitrate-activating enzyme(s) has been intensely studied for over 30 years, generating a host of candidates and a mountain of confusing and often contradictory data. Recently, the work of Chen et al. (2002) has sparked a flurry of interest by convincingly showing that the enzyme, mitochondrial aldehyde dehydrogenase (mtADH), is a suitable candidate. Subsequent work has supported this proposal (Daiber et al., 2004; Sydow et al., 2004; Zhang et al., 2004; Kollau et al., 2005) and it has now been shown that mtADH meets most criteria of the physiological GTN-activating enzyme: it is an intracellular enzyme that is activated by low concentrations (nanomolar) of GTN; it contains active sulphydryl groups that are redox regulated; it generates the correct metabolites (1,2-glyceryl-dinitrate and NO) via an S-nitrosothiol or nitrite intermediate; it releases NO from physiologically active concentrations of GTN, inhibitors of mtADH inhibit 1,2-glyceryl-dinitrate production and vasodilatation and its activity is reduced with continuous use of GTN (summarized by Ignarro, 2002). In addition, transgenic mice lacking the mtADH genes do not have the high affinity pathway of GTN vasodilatation seen in wild-type mice (Chen et al., 2005). The latter two points are crucial, because the main limitation of the organic nitrates is the well-documented development of tolerance with prolonged continuous use (succinctly reviewed in Gori and Parker, 2002a, 2002b). The only reliable means to avoid tolerance is to incorporate a nitrate-free interval in the therapeutic regimen, which can be problematic for some forms of angina and is a clear impediment to the use of nitrates for management of chronic conditions. Additionally, in vivo studies in animals (Munzel et al., 1995, 1999, 2000) and humans (Sage et al., 2000) have shown that tolerance may be associated with endothelial dysfunction and enhanced oxidative stress. This may be a contributory factor in reports that long-term nitrate use causes a paradoxical increase in risk of cardiac events (Ishikawa et al., 1996; Nakamura et al., 1999). The underlying cause of tolerance and better methods of bypassing this problem might emerge once the mechanism of action of nitrates has been fully elucidated. Although the role of mtADH is compelling, contradictory findings have already been published (DiFabio et al., 2003; de la Lande et al., 2004) and a number of other important mechanistic questions remain, most notably: why is the activity of mtADH only altered by specific thiols and not others, for example glutathione (Ignarro 2002)? What chemical reactions allow the nitrate moiety from GTN to react with thiols and/or lead to NO production (Ignarro, 2002; Daiber et al., 2004)? Why does the nitrate pentaerythrityl tetranitrate (PETN; Figure 2c) cause less desensitization of the esterase activity of mtADH and is less susceptible to tolerance (Daiber et al., 2004)? Why do inhibitors of mtADH not inhibit all nitrates, for example ISDN (Daiber et al., 2004)? Does the cellular distribution of mtADH explain why nitrates are venoselective (Ignarro, 2002), if indeed they are (Sogo et al., 2000a; Miller et al., unpublished results). In the face of a wide range of NO donors that do not develop tolerance (Brilli et al., 1997; Miller et al., 2000) and are better antiplatelet agents (Sogo et al., 2000b), the prevalence of these drugs in the clinical setting may diminish in favour of other agents.
Figure 2.
Chemical structure of NO donor drugs used clinically: the organic nitrates (a–c) and sodium nitroprusside (d). The NO-containing moiety is shown in bold.
The only other notable advance with this class of drugs is the launch of BiDil (isosorbide dinitrate with hydralazine; Sica, 2006) in 2005 for use in heart failure in African Americans (Pukrein and Yancy, 2005). The development has revived interest in the arena, more for the controversy stirred up by its targeting at a specific racial group (Haga and Ginsburg, 2006) than for any particular novelty in the therapeutic approach.
The other clinically relevant NO donor in current use is sodium nitroprusside (SNP; Figure 2d). SNP is used on-site in hospitals to provide rapid lowering of blood pressure in hypertensive crises. SNP is also the drug of choice in clinical studies, where it is recognized as the gold standard NO-dependent, but endothelium-independent vasodilator, although the complex mechanism involved in NO release might indicate that some of the novel NO donors that are emerging might be better suited for this purpose.
The mechanism of NO release from SNP in biological tissue is more complex than is often assumed and has not yet been fully elucidated. Contrary to popular belief, SNP is relatively stable and does not release NO spontaneously in the physiological environment; instead, NO generation requires either light or a tissue-specific mode of release (Butler and Megson, 2002; Grossi and D'Angelo, 2005). Of particular concern with this NO donor is the potential for release of any of the five cyanide groups incorporated in the structure (Bates et al., 1991). Indeed, there have been isolated reports that long-term use of this agent can be associated with cases of cyanidosis, albeit rarely (see Butler and Glidewell, 1987). Further limitations for the use of SNP are its requirement for intravenous administration, sensitivity to photolysis once in solution and its remarkable potency, which can make dose titration difficult (Megson, 2000). Given these limitations, it is difficult to envisage a wider application of this NO donor drug.
Diazeniumdiolates (NONOates)
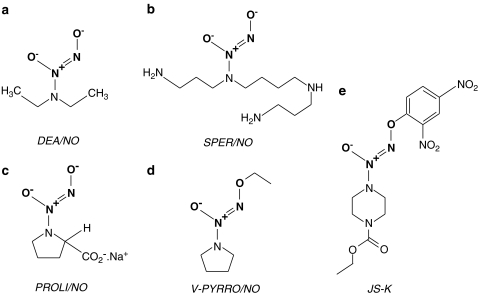
Diazeniumdiolates (also known as ‘NONOates') have been recognized for many years. The first of this class to be described was an adduct of diethylamine and NO (diethylamine NONOate; DEA/NO), first synthesized in 1960 (Drago and Paulik, 1960). However, diazeniumdiolates only became the focus of attention in the NO world in the 1990s, when their NO donor properties were considered in biological settings (Maragos et al., 1991). These compounds consist of a diolate group [N(O−)N=O] bound to a nucleophile adduct (a primary or secondary amine or polyamine) via a nitrogen atom (Maragos et al., 1991). NONOates decompose spontaneously in solution at physiological pH and temperature, to generate up to 2 molar equivalents of NO. The rate of decomposition is dependent on the structure of the nucleophile (Hrabie et al., 1993). A range of NONOates have now been described with half-lives varying from seconds to hours (Morley and Keefer, 1993). An attractive feature of this class of compounds is that their decomposition is not catalysed by thiols or biological tissue, unless specifically designed to (see below) and, because NO release follows simple first-order kinetics (Morley et al., 1993; Mooradian et al., 1995; Kavdia and Lewis, 2003), the rate of NO release can be accurately predicted. Subsequently, biological activity such as vasodilatation (Maragos et al., 1991; Morley et al., 1993), inhibition of platelet aggregation (Diodati et al., 1993; Sogo et al., 2000b), inhibition of blood coagulation (Nielsen, 2001) and inhibition of VSMC proliferation (Mooradian et al., 1995) closely correlate with the amount of NO generated in vitro. Additionally, the lack of tissue requirement for NO release is most likely responsible for the apparent lack of tolerance experienced with these compounds (Brilli et al., 1997).
At present, NONOates are not used clinically, although they have been tested frequently in experimental models of cardiovascular disease. For example, DEA/NO (Figure 3a) prevents and reverses vasospasm in a primate model of subarachnoid haemorrhage, without affecting systemic blood pressure (Pluta et al., 1997). Several different NONOates have been shown to lower pulmonary vascular resistance, with (Vanderford et al., 1994) or without (Brilli et al., 1997) decreasing systemic vascular resistance, depending on the design of the nucleophile adduct. Actions can be further improved by using formulations to aerosolize NONOates in combination with surfactants (Jacobs et al., 2000), making these drugs useful alternatives in the treatment of pulmonary hypertension or acute lung injury. NONOates may also have a use in the treatment of erectile dysfunction by enhancing blood flow to the penis (Talukdar and Wang, 2005), although it remains to be determined whether these drugs can be applied in a way that would have advantages over the sildenafil-type drugs.
Figure 3.
Chemical structure of five examples of the diazeniumdiolate (NONOate) class of NO donor drug. The diolate group (shown in bold) releases NO in solution, although prior cleavage of the molecule to release the terminal oxygen may be required (i.e. for V-PYRRO/NO and JS-K).
The primary cardiovascular focus for NONOates has been in the prevention of thrombosis and neointimal formation following vascular injury, an inevitable result of interventional cardiology techniques, such as balloon angioplasty, bypass grafting or placement of stents. Spermine NONOate (SPER/NO; Figure 3b), applied perivascularly, reduces neointimal formation induced by peri-arterial collars (Yin and Dusting, 1997), balloon angioplasty (Kaul et al., 2000) and also in bypass veins (Chaux et al., 1998). MAHMA/NO-eluting stents also reduce platelet adhesion to artificial grafts (Hanson et al., 1995). Infusion of PROLI/NO (Figure 3c) decreases platelet deposition to downstream polyester vascular grafts in the baboon without affecting mean arterial pressure (Saavedra et al., 1996). Local infusion devices releasing PROLI/NO also reduce cell proliferation following endarterectomy-induced injury (Chen et al., 1997). PROLI/NO, and other NONOates have been incorporated into an insoluble solid matrix that can be used to coat surfaces such as glass (Saavedra et al., 1996) and polymer films (Mowery et al., 2000) to minimize the thrombogenecity of localized delivery devices, extracorporeal circuits and blood pressure monitors. The same group have also synthesized a NONOate adduct of heparin that is still able to inhibit thrombin-induced platelet aggregation, but has the advantage over conventional heparin in that it can also inhibit ADP-induced platelet aggregation (Saavedra et al., 2000a). However, this compound has only had limited success when incorporated into a polymer film (Mowery et al., 2000). NONOates have also been used to coat the external surface of oxygen-sensing electrodes to prevent thrombus formation on the sensor, which can interfere with measurements of blood gases and electrolytes (Schoenfisch et al., 2000).
Modification of the structure of the nucleophile adduct to protect the terminal oxygen of the diolate moiety can stabilize the drug in solution and potentially engender a selective NO release in different organs, vascular beds or specific cell types. For example, Saavedra et al. (1997) have made O-alkenyl/O-alkyl derivatives of existing NONOates (e.g. V-PYRRO/NO; Figure 3d) to target NO release in liver cells. Of five cell types tested, V-PYRRO/NO blocked tumour necrosis factor-α (TNF-α)-induced apoptosis only in hepatocytes and protected against experimentally induced liver toxicity in rats in vivo. Similarly, another NONOate prodrug was synthesized that was stable in solution, but released NO following esterase activity within cells, causing apoptosis of the human leukaemia cell lines studied (Saavedra et al., 2000b). The group have also used piperazine as a linker molecule to produce a fluorescent NONOate (GLO/NO), and a method whereby the NONOate group can be linked to nonsteroidal anti-inflammatory drugs (NSAIDs), vitamin B3 and polyethylene glycol (Saavedra et al., 1999). The use of these compounds in biological systems largely remains at a theoretical level at present.
The use of NONOate adducts has received interest from the viewpoint of targeting delivery of high concentrations of NO specifically to tumour cells. Wang's group (Tang et al., 2001) coupled PYRRO/NO to a chain of amino acids recognized to be a substrate for prostate-specific antigen (PSA). PSA is inactive in blood plasma, but upregulated within prostate cancer metastases. This conjugated NONOate did not release NO in solution in the absence of PSA, offering the possibility that NO release will occur exclusively within cancer cells rather than in the blood. The same group have also synthesized sugar-conjugated NONOates, which they envisage will be preferentially taken up by cancer cells that overexpress the GLUT-1 transporter (Wu et al., 2001). A NONOate group has also been attached to the frequently used antitumour agent, 5-fluorouracil, engendering greater cytotoxicity on the prostate and cancer cells tested (Cai et al., 2003). More recently, the group has described the antitumour activity of a β-galactosyl-linked NONOate (Chen et al., 2006a), although the authors note that for clinical purposes, gene therapy to transfect tumour cells with LacZ would be required before drug administration. The same compound was also a highly efficient antibacterial agent (Chen et al., 2006b). JS-K (Figure 3e), a terminal oxygen-protected NONOate, has been developed by the US National Cancer Institute (NCI). The glutathione S-transferase enzymes are responsible for cleaving the compound to expose the diolate group and release NO, but despite the ubiquitous nature of these enzymes, JS-K slows the growth of cancer cells without harming healthy cells (Shami et al., 2003). Since being accepted as part of the NCI's ‘Rapid Access to Interventional Development (RAID) programme, JS-K has been shown to be active against a wide range of cancer cells (Cai et al., 2005).
We are currently aware of only a single study using NONOates in humans (in patients with respiratory distress syndrome; Lam et al., 2002). However, the predictable nature of NO release from NONOates will undoubtedly lead to further clinical investigations, once long-term safety has been established. The toxicity of by-products needs to be more fully confirmed (Lam et al., 2003), especially as subsequent reactions between decomposition products could lead to the formation of carcinogenic nitrosamines (Maragos et al., 1991). Incorporation of NONOates into polymers may represent a means of preventing the leaching of by-products (Mowery et al., 2000). At present, conjugated NONOates hold a great deal of promise, especially for the treatment of certain cancers, although further characterization of these drugs is essential before they reach larger clinical trials. The potential for oral preparations of NONOates has yet to be fully clarified, although transdermal preparations have already been developed (Shabani et al., 2001). However, experimentally, the NONOates remain an invaluable scientific tool for researching NO physiology.
S-Nitrosothiols
The S-nitrosothiol class of NO donors covers a vast array of different compounds which contain a single chemical bond between a thiol (sulphydryl) group (R-SH) and the NO moiety. Biological activity of S-nitrosothiols is highly influenced by the molecular environment of the parent thiol. That said, the complex chemistry of NO release from even the most basic S-nitrosothiol gives these compounds several means by which they can confer NO bioactivity. For instance, S-nitrosothiols are considered to be NO+ donors (see below) and transfer of NO+ across the plasma membrane via protein disulphide isomerases (Zai et al., 1999) may allow even large molecule weight S-nitrosothiols to transfer oxides of nitrogen across cell membranes to subcellular targets. The complex chemistry of NO release from S-nitrosothiols is beyond the scope of this review and has been reviewed elsewhere (Williams, 1999; Al-Sa'doni and Ferro, 2000; Megson and Webb, 2002). However, it is important to acknowledge that a vast number of factors are capable of releasing NO from S-nitrosothiols, including light, heat, transition metals, thiols, superoxide and enzymes such as xanthine oxidase (Trujillo et al., 1998), superoxide dismutase (Jourd'heuil et al., 1999), protein disulphide isomerase (Ramachandran et al., 2001) and various dehydrogenases (Liu et al., 2001). Subsequently, S-nitrosothiols have advantages over other classes of NO donors, such as the nitrates, as they have far less stringent metabolic requirements and this may be the reason that they do not induce tolerance with long-term use (Hanspal et al., 2002; Shaffer et al., 1992; Miller et al., 2000).
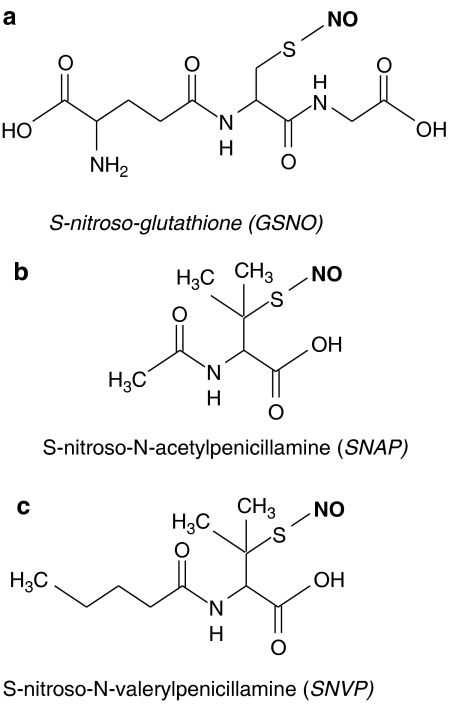
S-Nitrosothiols have a number of potential advantages over other classes of NO donor. Firstly, some examples show tissue selectivity: S-nitroso-glutathione (GSNO; Figure 4a) is selective for arteries over veins, giving them a different haemodynamic profile of action than those of classical organic nitrates. Additionally, S-nitrosothiols are potent antiplatelet agents, inhibiting aggregation at doses that do not influence vascular tone (de Belder et al., 1994; Ramsay et al., 1995). Furthermore, the ability of S-nitrosothiols to directly transfer NO+ species allows biological activity to be passed on through a chain of other thiols without the release of free NO. This mechanism of bioactivation may make S-nitrosothiols less susceptible to conditions of oxidative stress by effectively protecting the NO moiety from attack by oxygen-centred free radicals. It may be somewhat ambitious to suggest that S-nitrosothiols could also act as antioxidants conferred by the parent thiol, although GSNO has at least the potential to concurrently boost intracellular levels of the endogenous antioxidant, GSH. What is certain is that the endogenous nature of S-nitrosothiols such as GSNO (found at concentrations as high as 250 nM in some tissues; Bryan et al., 2004), coupled with in vitro evidence from high concentration, long-term incubations (20 h; Miller et al., 2000) and in vivo data (Shaffer et al., 1992), suggests that such S-nitrsothiols are unlikely to carry significant cytotoxicity and pharmacologically relevant concentrations.
Figure 4.
Chemical structure of S-nitrosothiols. The NO moiety is shown in bold.
S-Nitrosothiols are not used clinically at present, but there are a large number of animal and clinical studies demonstrating their advantageous features, especially in the cardiovascular system. GSNO has been shown to decrease the occurrence of cerebral embolism after carotid endarterectomy in patients already receiving aspirin and heparin (Molloy et al., 1998; Kaposzta et al., 2001). Additionally, GSNO reduces platelet adhesion in bypass grafts (Salas et al., 1998), thrombosis following coronary angioplasty (Langford et al., 1994) and emboli that dissociate from carotid plaques (Kaposzta et al., 2002). GSNO also shows beneficial haemodynamic effects in pre-eclampsia, without influencing fetal Doppler indices (Lees et al., 1996). A 2-week administration of S-nitroso-N-acetylcysteine, at a dose without vasomotor activity has been shown to more than halve lesion size in a transgenic mouse model of early atherosclerosis (Krieger et al., 2006). A 3-min intracoronary infusion of S-nitroso-N-acetyl-penicillamine (SNAP; Figure 4b) before the ischaemic period has been shown to decrease infarct size and improve coronary endothelial function (Gourine et al., 2002). S-nitrosoalbumin, a naturally occurring S-nitrosothiol, which can be formed from other NO donors (Crane et al., 2002), reduces platelet adhesion and neointimal thickening in angioplasty-damaged blood vessels, when given as an infusion (Marks et al., 1995) or as a stent-coating (Maalej et al., 1999).
The advantageous effects of S-nitrosoalbumin may be partly owing to its adhesive properties. We have carried out extensive experimentation on a group of novel S-nitrosothiols with alterations to their chemical structure to increase lipophilicity. We demonstrated that bolus injection of these lipophilic S-nitrosothiols produced a sustained vasodilatation (>4 h) in endothelium denuded arteries that was not seen with conventional S-nitrosothiols in isolated rat (Megson et al., 1997; Megson et al., 1999) and human (Sogo et al., 2000a) blood vessels, as well as human hand veins in vivo (Sogo et al., 2000c). We hypothesized that these novel compounds are retained in the lipophilic areas of the subendothelium where they slowly release NO over a prolonged period (Megson, 2002). Because the sustained effects were not seen in endothelium-intact arteries, these compounds mat be a way of targeting NO to areas of endothelial damage and, therefore, minimize side effects such as large sustained falls in blood pressure. Subsequently, we showed that one such compound, S-nitroso-N-valerylpenicillamine (SNVP; Figure 4c) inhibited platelet adhesion to rabbit carotid arteries damaged by balloon angioplasty in vivo suggesting a wider application of these drugs in vascular disease (Miller et al., 2003). Importantly, these novel compounds do not produce tolerance with long-term continuous use and remain fully activity in nitrate-tolerant blood vessels (Miller et al., 2000).
Outside the cardiovascular system, GSNO has been shown to have neuroprotective properties via regulation of antioxidant and apoptotic enzymes and, subsequently, may have a role in delaying progression of neurodegenerative disorders or even encouraging neuroregeneration (Rauhala et al., 2005). GSNO has also been shown to minimize liver deterioration when added to University of Wisconsin (UW) solution during cold storage during liver transplantation (Quintana et al., 2001). Supplementation of endogenous S-nitrosothiols may also encourage wound healing: Achuth et al. (2005) demonstrated in rats that systemically administered GSNO at concentrations that did not cause hypotension increased collagen deposition at sites of cutaneous incisions, without affecting matrix metalloproteinase/collagenolytic pathways. These results are encouraging for the development of dressings for local delivery of NO to wounds. A cell-permeable form of S-nitroso-cysteine has been synthesized which can inhibit intracellular superoxide formation in neutrophils (Clancy et al., 2001). S-Nitroso-albumin has also been shown to have several beneficial actions that reduce inflammation and pulmonary vascular remodelling following hypoxia and reoxygenation in a mouse model of sickle cell disease (de Franceschi et al., 2006).
Several new S-nitrosothiol drugs have been described in the past few years. A more stable analogue of GSNO, LA810 (Lacer, Barcelona, Spain) has been shown to have a marginally greater antithrombotic action than GSNO, in whole blood using a Badimon chamber as a model for ex vivo thrombus formation (Vilahur et al., 2004). A polyethylene glycol-conjugated form of S-nitroso-albumin has been described with improved distribution and prolonged NO release in the circulation (Katsumi et al., 2005). Finally, ‘fillers' have been designed to blend NO donors into polymers, allowing the release of NO but not other by-products (Frost and Meyerhoff, 2005). This publication also presents encouraging data showing that S-nitrosothiols such as SNAP can be sandwiched into the centre of a tri-layer of polymers for use as a film on medical devices. NO release could be ‘switched on and off' using light irradiation (or provision of Cu(I) ions) and levels controlled and maintained over 12 h by varying the design of the films. Importantly, NO is not liberated in plasma, suggesting the pool of NO would not be released until a trigger was provided.
In light of the preclinical data discussed above, S-nitrosothiols should have a promising future. The foundations for targeted delivery have already been provided and will likely be expanded upon, by incorporation of these drugs to devices for cardiological intervention. Despite cost implications, much research has been garnered into drug-eluting stents (Eisenberg, 2006; Ryan and Cohen, 2006) and the flexibility of S-nitrosothiols makes them ideal candidates for preventing in-stent thrombosis, at least in the short term. Manipulating chemical properties such as lipophilicity may provide targeted delivery of NO and different routes of administration, including transdermal preparations. Indeed, preliminary work has already been carried out using topical application of synthetic S-nitrosothiols to skin microvessels (Khan et al., 1997; Seabra et al., 2004).
NO hybrid drugs
Hybrid NO donor drugs represent a novel approach to the design of NO-releasing compounds. This is a broad grouping that covers a range of established drugs that have been structurally modified to incorporate NO-containing molecules. The aim of this strategy is to synthesize drugs that retain the pharmacological activity of the parent compound, but also have the biological actions of NO. Importantly, the release of NO must be balanced to provide sufficient activity within the concentration range of the parent compound (Bandarage et al., 2000).
NO-NSAIDs
The high efficacy and low cost of NSAIDs in the treatment of both mild and severe inflammatory conditions, have led to drugs such as aspirin becoming invaluable in a number of clinical fields. Subsequently, use of NSAIDs is commonplace for the treatment of persistent inflammation, such as rheumatism and arthritis. Low-dose aspirin is also routinely used prophylactically to reduce the risk of thrombotic events associated with a wide range of cardiovascular conditions. However, prolonged use of aspirin leads to serious side effects in the gastrointestinal tract that have been reported to cause ∼16 000 deaths each year in the USA (Keeble and Moore, 2002). Furthermore, a recent report highlights a further increase in the risk of upper gastrointestinal bleeding attributed to the co-administration of multiple antithrombotic therapies regularly prescribed for cardiovascular conditions (Hallas et al., 2006). NO has a number of effects in the gastrointestinal tract that could counteract the loss of protective prostanoids caused by aspirin. NO increases secretion of protective gastric mucus (Brown et al., 1993), increases blood flow to the gastric mucosa, promoting repair and removal of toxins (Hallas et al., 2006), decreases interaction of neutrophils with the gastric microcirculation (Wallace, 1997) and may also promote the healing of gastric ulcers (Ma and Wallace, 2000). Therefore, incorporating NO-releasing properties into a NSAID may minimize the gastric side effects of drugs such as aspirin.
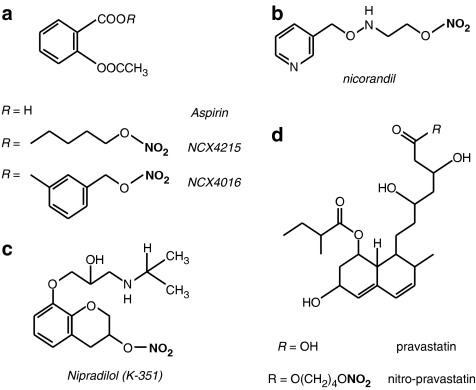
The first NO-NSAID compounds designed and released commercially were the NicOx compounds, NCX4016 and NCX4215 (Figure 5a). Both are derivatives of aspirin (often referred to as ‘nitroaspirins') adapted to contain a nitrate group. These compounds have been shown to retain the ability of aspirin to inhibit inflammation and nociception without causing gastric ulcers seen with equivalent concentrations of aspirin (del Soldato et al., 1999; Fiorucci et al., 2003; Turnbull et al., 2006b). Also, several studies have shown that these compounds have comparable or greater antiplatelet effects than the parent NSAID, without causing excessive vasodilatation or hypotension (Lechi et al., 1996; del Soldato et al., 1999; Wallace et al., 1999a; Momi et al., 2000). The biological actions of nitroaspirins have received much attention and have been reviewed extensively elsewhere (Keeble and Moore, 2002; Wallace et al., 2002; Chiroli et al., 2003; Wallace and Del Soldato, 2003; Turnbull et al., 2006b). Here, we will concentrate on a few new developments using nitroaspirins and then focus on emerging novel NO-NSAID compounds.
Figure 5.
Chemical structure of NO donor hybrid drugs containing a nitro-oxy moiety. The nitro-oxy group is shown in bold.
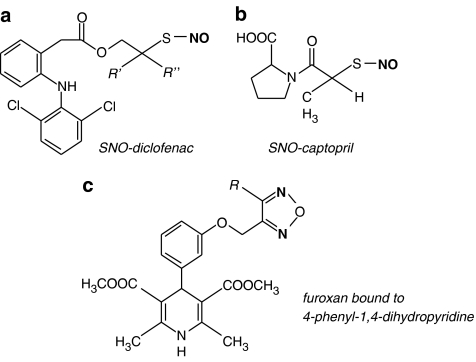
In the past 5 years, there has been considerable investigation into the mechanism that underpins the anti-inflammatory properties of NO generated from nitroaspirins (Keeble and Moore, 2002). Caspases are a family of proteases involved in cytokine release and apoptosis. NO from NCX4016 inhibits the action of capsase-1, and, subsequently, the propagation of other cytokines such as IL-1β and IL-8 (Fiorucci et al., 2000). NCX4016 induced inhibition of capsase-1 is mediated through S-nitrosylation of a sulphydryl group (Dimmeler et al., 1997), therefore, other NO-NSAIDs, such as S-nitroso-diclofenac (see Figure 6a) may be more effective inhibitors of capsase-1 through transnitrosation reactions. Nitroaspirins also inhibit the release of TNF-α from lipopolysaccharide-stimulated macrophages (Minuz et al., 2001), although it is difficult to determine whether this is a direct effect of NO or through inhibition of other cytokines. Regardless, this property would likely provide benefits in the treatments of a range of inflammatory diseases, above that of conventional aspirin (Fiorucci and Del Soldato, 2003).
Figure 6.
Chemical structure of NO donor hybrid groups containing a (a, b) S-nitrosothiol or a (c) furoxan moiety. The NO containing moiety is shown in bold.
NO-NSAIDs have attractive properties in a number of cardiovascular conditions. On top of the antiplatelet actions of NO-NSAIDs, the NO-mediated anti-inflammatory properties would be useful in vascular injury and atherosclerosis, given the central role of inflammatory cells in the process (Ross, 1999). NCX4016, but not aspirin, has been shown to reduce experimental restenosis in hypercholesterolemic (Napoli et al., 2001) and aged (Napoli et al., 2002) mice. NCX4016 also reduces infarct size in several different models of myocardial ischaemia-reperfusion injury (Rossoni et al., 2000, 2001; Wainwright et al., 2002; Burke et al., 2006) and has been shown to reduce platelet–monocyte interaction in humans to a greater extent than aspirin alone (Fiorucci et al., 2004).
Nitroaspirins also show potential in cancer therapy. Upregulation of cyclooxygenase-2 leading to enhanced prostaglandin output is a feature of a number of cancers (Baron, 1995). Both the gastro-sparing properties of nitroaspirins and the direct effect of NO on cell proliferation could be beneficial, although obtaining suitable balance between the different facets of a nitroaspirin might prove difficult. That said, NCX4016 have been shown to be 250–6000-fold more effective at inhibiting the growth of a number of different cancer cell lines (Kashfi and Rigas, 2005). Additionally, the compound also reduced tumour growth in an in vivo rat model of colonic adenocarcinoma to a greater extent than aspirin itself (Bak et al., 1998). Aside from cancer, NO-NSAIDs may have applications in other areas of the body (Keeble and Moore, 2002). A NO-releasing derivative of flurbiprofen has been shown to have beneficial actions in models of renal ablation (Fujihara et al., 1998), bone degeneration (Armour et al., 2001) and Alzheimer's disease (Jantzen et al., 2002).
Given the tolerance issues associated with organic nitrates, it is surprising that the majority of nitroaspirins investigated so far exploit the same NO donor moiety, especially as it has been shown for at least one example that the mechanism of NO release is the same in hybrid drugs (Turnbull et al., 2006a). This is a pressing concern considering the rapid emergence of many new NSAIDs/anti-inflammatory/analgesic agents with nitrates groups, for example paracetamol (Marshall et al., 2006), flurbiprofen (Fujihara et al., 1998), naproxen (Young et al., 2005), mesalamine (Wallace et al., 1999b), gabapentin (Wu et al., 2004), predisolone and other steroids (Tallet et al., 2002). However, the nitrate ester of nitroaspirin has been replaced with a furoxan moiety (Cena et al., 2003; Turnbull et al., 2006a) and S-nitroso- (Bandarage et al., 2000) and diazeniumdiolate (Velazquez et al., 2005) forms of other NSAID drugs (e.g. aspirin, diclofenac, indomethacin and ibuprofen) have also been described. These alternative forms of NO-NSAID has advantages over nitrate-linked NSAIDs in that they are likely to have less strict requirements for the release of NO and, therefore, may exert greater antiplatelet activity and would be unlikely to produce tolerance (Turnbull et al., 2006b).
Other nitrate-hybrid drugs
There are several other drugs of note that have been successfully adapted to contain a nitrate to provide NO bioactivity. The first, nicorandil (Figure 5b), a nicotinamide derivative with a nitrate group that has both NO donor and mitochondrial K+ATP channel-opening properties. Although existing organic nitrates are extremely effective at relieving the acute symptoms of angina, clinical trials have not found that nitrates improve outcome (Yusuf et al., 1988; GISSI-3 study group, 1994; ISIS-), indeed, they may even worsen long-term prognosis (Ishikawa et al., 1996; Nakamura et al., 1999). Nicorandil, however, has been shown to decrease coronary events in over 5000 patients with stable angina during a mean follow-up period of 1.6 years (IONA study group, 2002), and subsequently, this drug is beginning to overshadow classical nitrates in the minds of many physicians. The additional beneficial properties of nicorandil have been largely attributed its actions on K+ channels, causing dilatation of peripheral arteries and coronary resistance vessels, reducing the afterload of the heart (Simpson and Wellington, 2004; Herman and Moncada, 2005). This proposal is somewhat puzzling, as it is regularly argued that the reason behind organic nitrate effectiveness in the relief of angina, is their ability to selectively dilate peripheral veins over arteries (Kojda, 2000), and large coronary arteries over small (Winbury et al., 1969; Kurz et al., 1991). Should the dual action of nicorandil on preload and afterload be the reason for its success, it would suggest that other NO donors with more balanced arteriovenous selectivity may also be useful in the treatment of angina and ischaemic heart disease. Alternatively, actions other than those on blood vessels may be important; for example, encouragement of fibrinolysis by decreasing the activity of type-1 plasminogen activator inhibitor (PAI-1) (Sakamoto et al., 2004) or by preconditioning the heart against ischaemic episodes (Matsuo et al., 2003). Currently, it is unclear whether long-term use of nicorandil is restricted by the development of tolerance, like other nitrates, with some studies showing no cross-tolerance to GTN with respect to vasodilatation (Simpson and Wellington, 2004), whereas others suggest that, while peripheral vasodilatation is unaffected, tolerance develops to the anti-ischaemic preconditioning (Rajaratnam et al., 1999; Loubani and Galinanes, 2002). Replacement of the nitrate group of nicorandil with a furoxan group (see below) has been described, (Cai et al., 2005), that may circumvent the problems of tolerance.
Nipradilol (K-351; Figure 5c) is an orally active compound that was first described in the early 1980s as a nonspecific β-receptor antagonist containing a nitrate group (Uchida et al., 1983). It was hypothesized that combining a NO donor with an adrenoceptor antagonist would provide additional beneficial cardiovascular actions by counteracting the undesirable side effects of the individual drugs. Addition of the nitrate group instils the drug with vasodilator actions similar to those of GTN, as well as enhancing the β-antagonistic potency and, surprisingly, providing a degree of antagonism for α-receptors (Uchida et al., 1983). There is conflicting evidence as to whether nipradilol shows selectivity for conduit (Uchida et al., 1983) or resistance (Lamping and Bloom, 1995) arteries. More recently, Thakur et al., (2002) demonstrated that nipradilol inhibits the development of atherosclerotic lesions in a rabbit model of hypercholesterolemia and eNOS inhibition. Interestingly, the beneficial actions of nipradilol appear to be in part owing to increased eNOS expression (Jayachandran et al., 2001; Thakur et al., 2002), although a mechanism for this has yet to be established. As with the conventional organic nitrates, the development of tolerance with continuous use may limit nipradilol's effectiveness.
Lastly, it is worth briefly mentioning another type of nitrate hybrid drug that, while only preliminary experimental data have been published so far, will undoubtedly come under close scrutiny. Statins are highly effective inhibitors of 3-hydroxy-methylglutaryl CoA reductase that are used to lower cholesterol. They have had a dramatic impact on clinical outcome in a number of cardiovascular conditions. It is now clear that statins have a number of additional molecular mechanisms beyond lipid-lowering, that ultimately modulate inflammation and smooth muscle cell proliferation. Ongini et al., (2004) have synthesized nitrate-linked forms of pravastatin (Figure 5d) and fluvastatin, which they believe could complement the existing statin effects in conditions where there is endothelial dysfunction, such as diabetes and atherosclerosis. In vivo effects have yet to be tested, but experiments in cell lines showed that the NO-statins had a far greater ability to inhibit cell proliferation compared to their parent compound, and were also shown to decrease iNOS expression and activity (Ongini et al., 2004). A very recent contribution to work in this area has been made by Dever et al. (2007) who have clearly shown a nitrate-derivative of pravastatin (NCX6550) to have significant inhibitory effects on ROS generation and adhesiveness in splenocytes from the Apo-E−/− murine model of atherosclerosis, as well as wild-type controls. NCX6550 was also shown to enhance endothelium-dependent vasodilatation in aortic rings from Apo-E−/− mice; of the benefits observed with NCX6550, only inhibition of ROS was shared by the parent compound, pravastatin.
Other S-nitroso-hybrid drugs
A hybrid approach has also been applied to inhibitors of angiotensin-converting enzyme (ACE). Captopril is an example of an ACE inhibitor that contains a SH group, which can be nitrosated, forming S-nitrosocaptopril (SNO-Cap; Figure 6b) (Loscalzo et al., 1989). SNO-Cap has sGC-mediated vasodilator and antiplatelet actions, yet retains the ability to inhibit ACE (Cooke et al., 1989; Loscalzo et al., 1989). Additionally, SNO-Cap appears to preferentially dilate coronary arteries over peripheral vessels (Cooke et al., 1989). Intravenous SNO-Cap produces a long-lasting hypotensive effect in vivo (Shaffer et al., 1991) and, similarly to other S-nitrosothiols, is less susceptible to tolerance (Shaffer et al., 1991; Zhang et al., 1994; Matsumoto et al., 1995). ACE inhibition may also contribute to the NO-mediated actions of SNO-Cap, as the enzyme also inactivates bradykinin, an endogenous endothelium-dependent vasodilator. Despite the obvious appeal of such a drug, there have been very few reports of SNO-Cap within the last 10 years, the most recent being a detailed study of NO release from SNO-Cap in the absence of tissue (Aquart and Dasgupta, 2004). This may be owing to the poor stability of SNO-Cap, making it difficult to purify and crystallize (Jia et al., 2000). In addition, several groups have been unable to replicate the synthesis of SNO-Cap and confirmation of the synthesis is required. That said, others have managed to synthesize red flake crystals of SNO-Cap and showed that these crystals have potent antiangiogenic effects in embryonic tissue (Jia et al., 2000), although it is not clear if conventional S-nitrosothiols would have a similar effect.
Tissue-type plasminogen activator (t-PA) is an endogenous enzyme synthesized by the endothelium. Fibrin, a constituent of thrombus, binds to t-PA stimulating the conversion of plasminogen into plasmin: a powerful fibrinolytic agent. t-PA contains a single SH group which can be S-nitrosated allowing t-PA to directly inhibit platelets as well as exerting a slightly greater fibrinolytic activity than native t-PA (Stamler et al., 1992). The combined antithrombotic and anti-inflammatory action of SNO-t-PA has been shown to reduce cardiac necrosis following ischaemia-reperfusion injury in vivo (Delyani et al., 1996). Von Willebrand factor (vWF) is synthesized and released by damaged blood vessels. The binding of vWF to platelet glycoprotein receptors induces platelet activation, causing adhesion to damaged regions of blood vessels. Recombinant fragments of vWF, such as AR545C, bind to platelet receptors, competing with the binding of endogenous vWF to platelet receptors and therefore preventing the initiation of thrombosis. Inbal and co-workers have shown that S-nitrosated AR545C causes greater inhibition of platelet adhesion and aggregation than recombinant vWF that is not S-nitrosated (Inbal, 1999). As far as we are aware, the application of SNO-t-PA and SNO-vWF has not been taken further, although the authors remain convinced of their therapeutic potential and aim to readdress these compounds within the near future (J Loscalzo; personal communication).
Other NO-hybrid drugs
Furoxans are a group of compounds that has been of considerable interest to chemists for decades, yet have received relatively little attention from biologists, despite their NO-releasing properties. The complicated chemistry of NO release from the many different compounds in this class are reviewed elsewhere (Gasco and Schoenafinger, 2005), here we focus on existing pharmacological agents that can be modified to contain the pentavalent furoxan group.
The dihydropyridine class of calcium antagonists can be linked a furoxan group (Figure 6c) and these compounds cause vasodilatation through sGC stimulation as well as inhibition of voltage-dependent Ca2+ ion channels on VSMCs (Di Stilo et al., 1998). The same group have also linked furoxans to α1- (Fruttero et al., 1995) and β1-antagonists (Boschi et al., 1997). Both produce vasodilatation of isolated aortic strips through a combination of adrenoceptor antagonism and NO release. A range of furoxan (and nitrated) phenols have also been synthesized, to provide a balanced NO-mediated vasodilatory response with antioxidant defence, with the underlying goal of treating atherosclerosis (Boschi et al., 2006).
Outside the cardiovascular system, furoxans have also been linked to a histamine H2 receptor antagonist, and provide a greater protection against gastric ulcers than antihistamine drugs alone (Coruzzi et al., 2000). Furoxan-linked H3-antagonists have also been described (Bertinaria et al., 2003b). Protein pump inhibitors (PPIs) have been a major success in a range of gastric pathologies. A furoxan form of the PPI, rabeprazole, has been shown to reduce histamine secretion and indomethacin-induced gastric lesion development in rats in vivo (Sorba et al., 2003). Remaining in the gastrointestinal system, furoxan derivatives linked to the antimicrobial drug metronidazole have been shown to have potent activity against Helicobacter pylori in metronidazole-resistant strains, although it is unclear if this property is owing to NO release per se (Sorba et al., 2003; Bertinaria et al., 2003a). Lastly, a uroselective furoxan linked α1-antagonist, REC15/2739, has a dual vasodilator action on noradrenaline-contracted rat vas deferens, that may be of benefit in the treatment of conditions such as benign prostatic hyperplasia (Boschi et al., 2003).
Zeolites
A novel approach to storage and delivery of NO has recently been adopted using ion-exchanged zeolites (Wheatley et al., 2006). These microporous insoluble materials form a framework containing metal ions that can bind NO. Exposure of the solid to NO gas results in NO binding to the metal ions within the pores, facilitating highly efficient packing of NO within the solid. NO-zeolites of this type are very stable in the anhydrous state, but NO is displaced by water on immersion in an aqueous environment. The beauty of these materials is that they constitute very high capacity stores for NO and the rate of release can be modulated by altering the porosity of zeolite, the metal ion in the framework and the composition and nature of the binder (Frost et al., 2005; Wheatley et al., 2006). This infinite flexibility with respect to NO release allows for development of a range of different NO donor materials for different purposes ranging from fast-acting antimicrobial coatings for urinary catheters and wound dressings to durable, slow acting antithrombotic coatings for stents, bypass tubing, cannulae and catheters. Although at an early stage, this approach represents a novel means of site-selective delivery of NO that optimizes the benefits of NO as a local mediator.
Summary and conclusions
Depression of the NO:sGC pathway is a feature of many cardiovascular conditions and delivery of low concentrations of exogenous NO is an attractive therapeutic option. In contrast, delivery of high concentrations might have completely different therapeutic targets and act via the cytotoxic actions of NO. The chemical versatility of NO has led to the synthesis of a wide range of NO donors, each with different modes and rates of NO release. Subsequently, NO donor drugs can be chosen or tailor-made to suit the disease target. Long-term use of current NO donors is limited by development of tolerance and toxicity issues, ensuring that there is a clinical need for novel alternatives.
NONOates have proved extremely popular in the experimental setting due to the predictable nature of NO release. However, use of these compounds should not be restricted to the bench; NONOates have been successfully applied in a number of cardiovascular conditions, especially where a slow prolonged release rate is desirable. In pulmonary hypertension, for example, NONOates are less likely to cause rebound hypertension on cessation than inhaled NO (Butler and Russell, 2005). There has been an explosion of interest in the possibility of delivering cytotoxic levels of NO to cancer cells to promote tumour regression. Subsequently, a series of NONOates have been designed with protected diolate groups that release NO upon metabolism by factors specific to tumour cells. However, the potential toxicity of by-products of NONOate metabolism still remains to be fully investigated before the clinical potential of these compounds can be fully assessed.
S-nitrosothiols are another group of NO donors that can be structurally modified to release NO at varying rates in specific conditions. Alterations of the structure of S-nitrosothiols to modify lipophilicity may allow us to target these compounds to areas of endothelial damage (Megson et al., 1997; Miller et al., 2003) and, therefore, minimize side effects. Both S-nitrosothiols and NONOates hold a great deal of promise as coatings for stents, extracorporeal circuits and catheters used in interventional cardiological procedures, surgery and renal replacement therapy. Polymeric materials are used to coat many such devices and all currently induce biological response (Frost et al., 2005). NO donor containing polymers will likely reduce a number of unwanted complications such as thrombosis and restenosis without the need for systemic administration of heparin or potent antiplatelet agents (Annich et al., 2000). Further research is now required to design agents that can release active concentrations of NO over a significant period of time.
Finally, the development of hybrid NO donor drugs has increased exponentially in recent years. The concept is best described by NO-NSAIDs that retain the actions of their parent compound (analgesia, inhibition of inflammation, decreased thrombosis), yet have additional beneficial actions attributed to NO, most notably in protecting against gastric damage caused by aspirin. Results from the use of NO-NSAIDs in animal models are promising but it remains to be seen whether the progress of these drugs is hindered or enhanced by the recent high-profile withdrawal of cyclooxygenase-2 specific inhibitors (Fitzgerald, 2004; Garcia Rodriguez et al., 2004). Regardless, the concept has been extended to a vast array of pharmacological agents such as anticoagulants, antitumour agents, ion channel inhibitors, ACE inhibitors and histamine receptor inhibitors. It remains to be seen whether use of these agents in clinical trials is more practical or have greater effects than co-administering individual drugs or using combinations tablets (Boschi et al., 2006; Turnbull et al., 2006b).
It has transpired that the development of a superior NO donor drug to rival convential organic nitrates has proved highly elusive. Despite the obvious potential, there have been very few notable advances in terms of licenced medicines that have emerged from the euphoria surrounding its identification as an important biological messenger. Indeed, far more progress has been made in targeting downstream sGC and cGMP, most notably with sildenafil (Sawatzky et al., 2005), which is likely to have many cardiovascular benefits beyond its well-documented application in erectile dysfunction. Indeed, it is fair to say that these downstream mechanisms might represent a more user-friendly means of systemic modulation of the NO pathway, whereas the ability to release NO itself may be either be too simple and/or rapid, leading to instability of the parent compound and lack of specificity, or too complex, resulting in tolerance associated with enzyme-mediated processes.
In assessing where developments might realistically emerge in the future, it is perhaps worth reconsidering the endogenous roles of NO, where it acts primarily as a local mediator. In vivo, the short biological half-life is exploited to facilitate local effects in response to specific stimuli; NO then simply dissipates through diffusion and oxidation to nitrite and nitrate, without the need for complex metabolism. Technologies that utilize this inherent special quality of NO have a great chance of success and a number of developments in this area have already begun to emerge, as described above. Wound healing is a particularly interesting target, given the multiple benefits that NO might have, from antimicrobial agent, through vasodilator and pro- or anti-inflammatory agent, depending on the concentration of NO generated and experimental materials (Masters et al., 2002) have shown some benefit in this setting. However, central to the success or otherwise of this (Schulz and Stechmiller, 2006) or any other NO donor therapy is generation of the correct amount of NO in the right place for the right length of time; cracking these problems will undoubtedly lead to a new range of NO donors that successfully exploit specific elements of the wide range of physiological roles played by this critical endogenous mediator.
Acknowledgments
Dr Miller is funded by a British Heart Foundation Programme Grant (RG/05/003).
Abbreviations
- ACE
angiotensin-converting enzyme
- DEA/NO
diethylamine NONOate
- EDHF
endothelium-dependent hyperpolarizing factor
- GSNO
S-nitroso-glutathione
- GTN
glyceryl trinitrate
- ISMN
isosorbide mononitrate
- mtADH
mitochondrial aldehyde dehydrogenase
- NCI
National Cancer Institute
- NO
nitric oxide
- eNOS
nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- NSAID
nonsteroidal anti-inflammatory drug
- PETN
pentaerythrityl tetranitrate
- PPI
protein pump inhibitor
- PSA
prostate-specific antigen
- sGC
soluble guanylate cyclase
- SNAP
S-nitroso-N-acetylpenicillamine
- SNO-Cap
S-nitroso-captopril
- SNP
sodium nitroprusside
- SNVP
S-nitroso-N-valerylpenicillamine
- SPER/NO
spermine NONOate
- TNF-α
tumour necrosis factor-α
- t-PA
tissue-type plasminogen activator
- VSMC
vascular smooth muscle cell
- vWF
von Willebrand factor
Conflict of interest
ILM held a consultancy at Strakan Pharmaceuticals (2003–2005).
MRM has no conflict of interest.
References
- Achuth HN, Moochhala SM, Mahendran R, Tan WT. Nitrosoglutathione triggers collagen deposition in cutaneous wound repair. Wound Repair Regen. 2005;13:383–389. doi: 10.1111/j.1067-1927.2005.130405.x. [DOI] [PubMed] [Google Scholar]
- Al-Sa'doni HH, Ferro A. S-Nitrosothiols: a class of nitric oxide-donor drugs. Clin Sci (Lond) 2000;98:507–520. [PubMed] [Google Scholar]
- Annich GM, Meinhardt JP, Mowery KA, Ashton BA, Merz SI, Hirschl RB, et al. Reduced platelet activation and thrombosis in extracorporeal circuits coated with nitric oxide release polymers. Crit Care Med. 2000;28:915–920. doi: 10.1097/00003246-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Aquart DV, Dasgupta TP. Dynamics of interaction of vitamin C with some potent nitrovasodilators, S-nitroso-N-acetyl-D,L-penicillamine (SNAP) and S-nitrosocaptopril (SNOCap), in aqueous solution. Biophys Chem. 2004;107:117–131. doi: 10.1016/j.bpc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Armour KJ, van't Hof RJ, Armour KE, Torbergsen AC, Del Soldato P, Ralston SH. Inhibition of bone resorption in vitro and prevention of ovariectomy-induced bone loss in vivo by flurbiprofen nitroxybutylester (HCT1026) Arthritis Rheum. 2001;44:2185–2192. doi: 10.1002/1529-0131(200109)44:9<2185::aid-art372>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bak AW, McKnight W, Li P, Del Soldato P, Calignano A, Cirino G, et al. Cyclooxygenase-independent chemoprevention with an aspirin derivative in a rat model of colonic adenocarcinoma. Life Sci. 1998;62:367–373. doi: 10.1016/s0024-3205(98)00191-x. [DOI] [PubMed] [Google Scholar]
- Bandarage UK, Chen L, Fang X, Garvey DS, Glavin A, Janero DR, et al. Nitrosothiol esters of diclofenac: synthesis and pharmacological characterization as gastrointestinal-sparing prodrugs. J Med Chem. 2000;43:4005–4016. doi: 10.1021/jm000178w. [DOI] [PubMed] [Google Scholar]
- Baron JA. Aspirin and cancer. Prev Med. 1995;24:121–124. doi: 10.1006/pmed.1995.1023. [DOI] [PubMed] [Google Scholar]
- Bates JN, Baker MT, Guerra R, Jr, Harrison DG. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem Pharmacol. 1991;42 Suppl:S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- Bath PM. The effect of nitric oxide-donating vasodilators on monocyte chemotaxis and intracellular cGMP concentrations in vitro. Eur J Pharmacol. 1993;45:53–58. doi: 10.1007/BF00315350. [DOI] [PubMed] [Google Scholar]
- Beltran B, Mathur A, Duchen MR, Erusalimsky JD, Moncada S. The effect of nitric oxide on cell respiration: a key to understanding its role in cell survival or death. Proc Natl Acad Sci USA. 2000;97:14602–14607. doi: 10.1073/pnas.97.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BM, Leitman DC, Schroder H, Kawamoto JH, Nakatsu K, Murad F. Relationship between biotransformation of glyceryl trinitrate and cyclic GMP accumulation in various cultured cell lines. J Pharmacol Exp Ther. 1989;250:316–323. [PubMed] [Google Scholar]
- Bertinaria M, Galli U, Sorba G, Fruttero R, Gasco A, Brenciaglia MI, et al. Synthesis and Anti-Helicobacter pylori properties of NO-donor/metronidazole hybrids and related compounds. Drug Dev Res. 2003a;60:225–239. [Google Scholar]
- Bertinaria M, Stilo AD, Tosco P, Sorba G, Poli E, Pozzoli C, et al. 3-(1H-imidazol-4-yl)propyl]guanidines containing furoxan moieties: a new class of H3-antagonists endowed with NO-donor properties. Bioorg Med Chem. 2003b;11:1197–1205. doi: 10.1016/s0968-0896(02)00651-x. [DOI] [PubMed] [Google Scholar]
- Boschi D, Di Stilo A, Cena C, Lolli M, Fruttero R, Gasco A. Studies on agents with mixed NO-dependent vasodilating and beta-blocking activities. Pharm Res. 1997;14:1750–1758. doi: 10.1023/a:1012136030849. [DOI] [PubMed] [Google Scholar]
- Boschi D, Tron GC, Di Stilo A, Fruttero R, Gasco A, Poggesi E, et al. New potential uroselective NO-donor α1-antagonists. J Med Chem. 2003;46:3762–3765. doi: 10.1021/jm030825u. [DOI] [PubMed] [Google Scholar]
- Boschi D, Tron GC, Lazzarato L, Chegaev K, Cena C, Di Stilo A, et al. NO-donor phenols: a new class of products endowed with antioxidant and vasodilator properties. J Med Chem. 2006;49:2886–2897. doi: 10.1021/jm0510530. [DOI] [PubMed] [Google Scholar]
- Brilli RJ, Krafte-Jacobs B, Smith DJ, Roselle D, Passerini D, Vromen A, et al. Intratracheal instillation of a novel NO/nucleophile adduct selectively reduces pulmonary hypertension. J Appl Physiol. 1997;83:1968–1975. doi: 10.1152/jappl.1997.83.6.1968. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochem Biophys Acta. 2004;1658:44–49. doi: 10.1016/j.bbabio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Brown JF, Keates AC, Hanson PJ, Whittle BJ. Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am J Physiol. 1993;265:G418–G422. doi: 10.1152/ajpgi.1993.265.3.G418. [DOI] [PubMed] [Google Scholar]
- Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, et al. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci USA. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgaud JL, Ongini E, Del Soldato P. Nitric oxide-releasing drugs: a novel class of effective and safe therapeutic agents. Ann NY Acad Sci. 2002;962:360–371. doi: 10.1111/j.1749-6632.2002.tb04080.x. [DOI] [PubMed] [Google Scholar]
- Burke SG, Wainwright CL, Vojnovic I, Warner T, Watson DG, Furman BL. The effect of NCX4016 [2-acetoxy-benzoate 2-(2-nitroxymethyl)-phenyl ester] on the consequences of ischemia and reperfusion in the streptozotocin diabetic rat. J Pharmacol Exp Ther. 2006;316:1107–1114. doi: 10.1124/jpet.105.096339. [DOI] [PubMed] [Google Scholar]
- Butler AR, Glidewell C. Recent chemical studies of sodium nitroprusside relevant to its hypotensive action. Chem Soc Rev. 1987;16:361–380. [Google Scholar]
- Butler AR, Megson IL. Non-heme iron nitrosyls in biology. Chem Rev. 2002;102:1155–1166. doi: 10.1021/cr000076d. [DOI] [PubMed] [Google Scholar]
- Butler AR, Russell JP.Vasodilators for biological research Nitric Oxide Donors 2005Wiley-VCH: Weihelm; 203–231.In: Wang PG, Cai TB, Taniguchi N (eds) [Google Scholar]
- Cai TB, Tang X, Nagorski J, Brauschweiger PG, Wang PG. Synthesis and cytotoxicity of 5-fluorouracil/diazeniumdiolate conjugates. Bioorg Med Chem. 2003;11:4971–4975. doi: 10.1016/j.bmc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Cai TB, Wang PG, Holder AA.NO and NO donors Nitric Oxide Donors 2005Wiley-VCH: Weinheim; 3–31.In: Wang PG, Cai TB, Taniguchi N (eds) [Google Scholar]
- Calver A, Collier J, Moncada S, Vallance P. Effect of local intra-arterial NG-monomethyl-L-arginine in patients with hypertension: the nitric oxide dilator mechanism appears abnormal. J Hypertens. 1992a;10:1025–1031. [PubMed] [Google Scholar]
- Calver A, Collier J, Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992b;90:2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cena C, Lolli ML, Lazzarato L, Guaita E, Morini G, Coruzzi G, et al. Antiinflammatory, gastrosparing, and antiplatelet properties of new NO-donor esters of aspirin. J Med Chem. 2003;46:747–754. doi: 10.1021/jm020969t. [DOI] [PubMed] [Google Scholar]
- Chaux A, Ruan XM, Fishbein MC, Ouyang Y, Kaul S, Pass JA, et al. Perivascular delivery of a nitric oxide donor inhibits neointimal hyperplasia in vein grafts implanted in the arterial circulation. J Thorac Cardiovasc Surg. 1998;115:604–612. doi: 10.1016/S0022-5223(98)70325-3. [DOI] [PubMed] [Google Scholar]
- Chen C, Hanson SR, Keefer LK, Saavedra JE, Davies KM, Hutsell TC, et al. Boundary layer infusion of nitric oxide reduces early smooth muscle cell proliferation in the endarterectomized canine artery. J Surg Res. 1997;67:26–32. doi: 10.1006/jsre.1996.4915. [DOI] [PubMed] [Google Scholar]
- Chen C, Shi Y, Li S, Qi Q, Gu L, Song J, Wang PG. A glycosylated nitric oxide donor, beta-Gal-NONOate, and its site-specific antitumor activity. Arch Pharm (Weinheim) 2006a;339:366–371. doi: 10.1002/ardp.200500262. [DOI] [PubMed] [Google Scholar]
- Chen C, Shi YQ, Song J, Qi QS, Gu L, Wang PG. Delivery of nitric oxide released from beta-Gal-NONOate activation by beta-galactosidase and its activity against Escherichia coli. Biol Pharm Bull. 2006b;29:1239–1241. doi: 10.1248/bpb.29.1239. [DOI] [PubMed] [Google Scholar]
- Chen Z, Foster MW, Zhang J, Mao L, Rockman HA, Kawamoto T, et al. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc Natl Acad Sci USA. 2005;102:12159–12164. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci USA. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiroli V, Benedini F, Ongini E, Del Soldato P. Nitric oxide-donating non-steroidal anti-inflammatory drugs: the case of nitroderivatives of aspirin. Eur J Med Chem. 2003;38:441–446. doi: 10.1016/s0223-5234(03)00055-2. [DOI] [PubMed] [Google Scholar]
- Clancy R, Cederbaum AI, Stoyanovsky DA. Preparation and properties of S-nitroso-L-cysteine ethyl ester, an intracellular nitrosating agent. J Med Chem. 2001;44:2035–2038. doi: 10.1021/jm000463f. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Andon N, Loscalzo J. S-nitrosocaptopril. II. Effects on vascular reactivity. J Pharmacol Exp Ther. 1989;249:730–734. [PubMed] [Google Scholar]
- Coruzzi G, Adami M, Morini G, Pozzoli C, Cena C, Bertinaria M, et al. Antisecretory and gastroprotective activities of compounds endowed with H2 antagonistic and nitric oxide (NO) donor properties. J Physiol Paris. 2000;94:5–10. doi: 10.1016/s0928-4257(99)00109-6. [DOI] [PubMed] [Google Scholar]
- Crane MS, Ollosson R, Moore KP, Rossi AG, Megson IL. Novel role for low molecular weight plasma thiols in nitric oxide-mediated control of platelet function. J Biol Chem. 2002;277:46858–46863. doi: 10.1074/jbc.M208608200. [DOI] [PubMed] [Google Scholar]
- Crane MS, Rossi AG, Megson IL. A potential role for extracellular nitric oxide generation in cGMP-independent inhibition of human platelet aggregation: biochemical and pharmacological considerations. Br J Pharmacol. 2005;144:849–859. doi: 10.1038/sj.bjp.0706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta E, Koshland DE., Jr NO news is good news. Science. 1992;258:1862–1865. doi: 10.1126/science.1361684. [DOI] [PubMed] [Google Scholar]
- Daiber A, Oelze M, Coldewey M, Bachschmid M, Wenzel P, Sydow K, et al. Oxidative stress and mitochondrial aldehyde dehydrogenase activity: a comparison of pentaerythritol tetranitrate with other organic nitrates. Mol Pharmacol. 2004;66:1372–1382. doi: 10.1124/mol.104.002600. [DOI] [PubMed] [Google Scholar]
- de Belder AJ, MacAllister R, Radomski MW, Moncada S, Vallance PJ. Effects of S-nitroso-glutathione in the human forearm circulation: evidence for selective inhibition of platelet activation. Cardiovasc Res. 1994;28:691–694. doi: 10.1093/cvr/28.5.691. [DOI] [PubMed] [Google Scholar]
- de Franceschi L, Malpeli G, Scarpa A, Janin A, Muchitsch EM, Roncada P, et al. Protective effects of S-nitrosoalbumin on lung injury induced by hypoxia-reoxygenation in mouse model of sickle cell disease. Am J Physiol Lung Cell Mol Physiol. 2006;291:L457–L465. doi: 10.1152/ajplung.00462.2005. [DOI] [PubMed] [Google Scholar]
- de la Lande IS, Stepien JM, Philpott AC, Hughes PA, Stafford I, Horowitz JD. Aldehyde dehydrogenase, nitric oxide synthase and superoxide in ex vivo nitrate tolerance in rat aorta. Eur J Pharmacol. 2004;496:141–149. doi: 10.1016/j.ejphar.2004.06.010. [DOI] [PubMed] [Google Scholar]
- del Soldato P, Sorrentino R, Pinto A. NO-aspirins: a class of new anti-inflammatory and antithrombotic agents. Trends Pharmacol Sci. 1999;20:319–323. doi: 10.1016/s0165-6147(99)01353-x. [DOI] [PubMed] [Google Scholar]
- Delyani JA, Nossuli TO, Scalia R, Thomas G, Garvey DS, Lefer AM. S-nitrosylated tissue-type plasminogen activator protects against myocardial ischemia/reperfusion injury in cats: role of the endothelium. J Pharmacol Exp Ther. 1996;279:1174–1180. [PubMed] [Google Scholar]
- Dever G, Spickett CM, Kennedy S, Rush C, Tennant G, Monopoli A, et al. The nitric oxide-donating pravastatin derivative, NCX6550 [(1S-[1-a(bS*, bS*), 2a, 6a, 8b-(R*), 8aa]]-1, 2, 6, 7, 8, 8a-hexahydro-b, d, 6-trihydroxy-2-methyl-8-(2-mehtyl-1-oxobutoxy)-1-napthalene-heptanoic acid 4-nitrooxy)butyl ester)], reduces splenocyte adhesion and reactive oxygen species generation in normal and atherosclerotic mice. J Pharmacol Exp Ther. 2007;320:419–426. doi: 10.1124/jpet.106.109298. [DOI] [PubMed] [Google Scholar]
- Di Stilo A, Visentin S, Cena C, Gasco AM, Ermondi G, Gasco A. New 1,4-dihydropyridines conjugated to furoxanyl moieties, endowed with both nitric oxide-like and calcium channel antagonist vasodilator activites. J Med Chem. 1998;41:5393–5401. doi: 10.1021/jm9803267. [DOI] [PubMed] [Google Scholar]
- DiFabio J, Ji Y, Vasiliou V, Thatcher GR, Bennett BM. Role of mitochondrial aldehyde dehydrogenase in nitrate tolerance. Mol Pharmacol. 2003;64:1109–1116. doi: 10.1124/mol.64.5.1109. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Haendeler J, Nehls M, Zeiher AM. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J Exp Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diodati JG, Quyyumi AA, Hussain N, Keefer LK. Complexes of nitric oxide with nucleophiles as agents for the controlled biological release of nitric oxide: antiplatelet effect. Thromb Haemost. 1993;70:654–658. [PubMed] [Google Scholar]
- Drago RS, Paulik FE. The reaction of nitrogen(II) oxide with diethylamine. J Am Chem Soc. 1960;82:96–98. [Google Scholar]
- Drexler H, Zeiher AM. Endothelial function in human coronary arteries in vivo. Focus on hypercholesterolemia. Hypertension. 1991;18:II90–II99. doi: 10.1161/01.hyp.18.4_suppl.ii90. [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ. Drug-eluting stents: the price is not right. Circulation. 2006;114:1745–1754. doi: 10.1161/CIRCULATIONAHA.106.646190. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Fenton C, Wellington K, Easthope SE. 0.4% nitroglycerin ointment: in the treatment of chronic anal fissure pain. Drugs. 2006;66:343–349. doi: 10.2165/00003495-200666030-00006. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Del Soldato P. NO-aspirin: mechanism of action and gastrointestinal safety. Dig Liver Dis. 2003;35 Suppl 2:S9–S19. doi: 10.1016/s1590-8658(03)00047-1. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Mencarelli A, Meneguzzi A, Lechi A, Renga B, Del Soldato P, et al. Co-administration of nitric oxide-aspirin (NCX-4016) and aspirin prevents platelet and moncyte activation and protects against gastric damage induced by aspirin in humans. J Am Coll Cardiol. 2004;44:635–641. doi: 10.1016/j.jacc.2004.03.079. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Santucci L, Cirino G, Mencarelli A, Familiari L, Soldato PD, et al. IL-1 beta converting enzyme is a target for nitric oxide-releasing aspirin: new insights in the antiinflammatory mechanism of nitric oxide-releasing nonsteroidal antiinflammatory drugs. J Immunol. 2000;165:5245–5254. doi: 10.4049/jimmunol.165.9.5245. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Santucci L, Gresele P, Faccino RM, Del Soldato P, Morelli A. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology. 2003;124:600–607. doi: 10.1053/gast.2003.50096. [DOI] [PubMed] [Google Scholar]
- Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- Frost MC, Meyerhoff ME. Synthesis, characterization, and controlled nitric oxide release from S-nitrosothiol-derivatized fumed silica polymer filler particles. J Biomed Mater Res A. 2005;72:409–419. doi: 10.1002/jbm.a.30275. [DOI] [PubMed] [Google Scholar]
- Frost MC, Reynolds MM, Meyerhoff ME. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contacting medical devices. Biomaterials. 2005;26:1685–1693. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Fruttero R, Boschi D, Di Stilo A, Gasco A. The furoxan system as a useful tool for balancing ‘hybrids' with mixed alpha 1-antagonist and NO-like vasodilator activities. J Med Chem. 1995;38:4944–4949. doi: 10.1021/jm00025a012. [DOI] [PubMed] [Google Scholar]
- Fujihara CK, Malheiros DM, Donato JL, Poli A, De Nucci G, Zatz R. Nitroflurbiprofen, a new nonsteroidal anti-inflammatory, ameliorates structural injury in the remnant kidney. Am J Physiol. 1998;274:F573–F579. doi: 10.1152/ajprenal.1998.274.3.F573. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Varas-Lorenzo C, Maguire A, Gonzalez-Perez A. Nonsteroidal antiinflammatory drugs and the risk of myocardial infarction in the general population. Circulation. 2004;109:3000–3006. doi: 10.1161/01.CIR.0000132491.96623.04. [DOI] [PubMed] [Google Scholar]
- Gasco A, Schoenafinger K.The NO-releasing heterocycles Nitric Oxide Donors 2005Wiley-VCH: Weinheim; 131–175.In: Wang PG, Cai TB, Taniguchi N (eds) [Google Scholar]
- GISSI-3 Study Group GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell'infarto Miocardico. Lancet. 1994;343:1115–1122. [PubMed] [Google Scholar]
- Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signalling, cytoprotection and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- Greenough A. Inhaled nitric oxide in the neonatal period. Expert Opin Investig Drugs. 2000;9:1601–1609. doi: 10.1517/13543784.9.7.1601. [DOI] [PubMed] [Google Scholar]
- Gori T, Parker JD. Nitrate tolerance: a unifying hypothesis. Circulation. 2002a;106:2510–2513. doi: 10.1161/01.cir.0000036743.07406.53. [DOI] [PubMed] [Google Scholar]
- Gori T, Parker JD. The puzzle of nitrate tolerance: pieces smaller than we thought. Circulation. 2002b;106:2404–2408. doi: 10.1161/01.cir.0000036742.52907.91. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Bulhak AA, Gonon AT, Pernow J, Sjoquist PO. Cardioprotective effect induced by brief exposure to nitric oxide before myocardial ischemia-reperfusion in vivo. Nitric Oxide. 2002;7:210–216. doi: 10.1016/s1089-8603(02)00114-3. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol. 2004;287:L262–L268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- Goyal P, Kiran U, Chauhan S, Juneja R, Choudhary M. Efficacy of nitroglycerin inhalation in reducing pulmonary arterial hypertension in children with congenital heart disease. Br J Anaesth. 2006;97:208–214. doi: 10.1093/bja/ael112. [DOI] [PubMed] [Google Scholar]
- Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353:2683–2695. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- Grossi L, D'Angelo S. Sodium nitroprusside: Mechanism of NO release mediated by sulfhydryl-containing molecules. J Med Chem. 2005;48:2622–2626. doi: 10.1021/jm049857n. [DOI] [PubMed] [Google Scholar]
- Haga SB, Ginsburg GS. Prescribing BiDil: is it black and white. J Am Coll Cardiol. 2006;48:12–14. doi: 10.1016/j.jacc.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Hallas J, Dall M, Andries A, Andersen BS, Aalykke C, Hansen JM, et al. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case–control study. Br Med J. 2006;333:726. doi: 10.1136/bmj.38947.697558.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SR, Hutsell TC, Keefer LK, Mooradian DL, Smith DJ. Nitric oxide donors: a continuing opportunity in drug design. Adv Pharmacol. 1995;34:383–398. doi: 10.1016/s1054-3589(08)61099-6. [DOI] [PubMed] [Google Scholar]
- Hanspal IS, Magid KS, Webb DJ, Megson IL. The effect of oxidative stress on endothelium-dependent and nitric oxide donor-induced relaxation: implications for nitrate tolerance. Nitric Oxide. 2002;6:263–270. doi: 10.1006/niox.2001.0412. [DOI] [PubMed] [Google Scholar]
- Herman AG, Moncada S. Therapeutic potential of nitric oxide donors in the prevention and treatment of atherosclerosis. Eur Heart J. 2005;26:1945–1955. doi: 10.1093/eurheartj/ehi333. [DOI] [PubMed] [Google Scholar]
- Hobbs AJ, Gladwin MT, Patel RP, Williams DL, Butler AR. Haemoglobin: NO transporter, NO inactivator or NOne of the above. Trends Pharmacol Sci. 2002;23:406–411. doi: 10.1016/s0165-6147(02)02067-9. [DOI] [PubMed] [Google Scholar]
- Hrabie JA, Klose JR, Wink DA, Keefer LK. New nitric oxide-releasing zwitterions derived from polyamines. J Org Chem. 1993;58:1472–1476. [Google Scholar]
- Ignarro LJ. After 130 years, the molecular mechanism of action of nitroglycerin is revealed. Proc Natl Acad Sci USA. 2002;99:7816–7817. doi: 10.1073/pnas.132271799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro LJ, Napoli C, Loscalzo J. Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: an overview. Circ Res. 2002;90:21–28. doi: 10.1161/hh0102.102330. [DOI] [PubMed] [Google Scholar]
- Inbal A. S-nitrosoderivative of a recombinant fragment of von Willebrand factor (S-nitroso-Ar545c): a unique antithrombotic agent. Isr Med Assoc J. 1999;1:290–291. [PubMed] [Google Scholar]
- IONA Study Group Effect of Nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet. 2002;359:1269–1275. doi: 10.1016/S0140-6736(02)08265-X. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Kanamasa K, Ogawa I, Takenaka T, Naito T, Kamata N, et al. Long-term nitrate treatment increases cardiac events in patients with healed myocardial infarction. Jpn Circ J. 1996;60:779–788. doi: 10.1253/jcj.60.779. [DOI] [PubMed] [Google Scholar]
- ISIS-4 Study Group ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet. 1995;345:669–685. [PubMed] [Google Scholar]
- Jacobs BR, Smith DJ, Zingarelli B, Passerini DJ, Ballard ET, Brilli RJ. Soluble nitric oxide donor and surfactant improve oxygenation and pulmonary hypertension in porcine lung injury. Nitric Oxide. 2000;4:412–422. doi: 10.1006/niox.2000.0292. [DOI] [PubMed] [Google Scholar]
- Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, et al. Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci. 2002;22:2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran M, Hayashi T, Sumi D, Thakur NK, Kano H, Ignarro LJ, et al. Up-regulation of endothelial nitric oxide synthase through beta(2)-adrenergic receptor-the role of a beta-blocker with NO-releasing action. Biochem Biophys Res Commun. 2001;280:589–594. doi: 10.1006/bbrc.2000.4177. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Rowe D, Emsley AM, Newby AC. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc Res. 1999;43:580–594. doi: 10.1016/s0008-6363(99)00171-6. [DOI] [PubMed] [Google Scholar]
- Jia L, Wu CC, Guo W, Young X. Antiangiogenic effects of S-nitrosocaptopril crystals as a nitric oxide donor. Eur J Pharmacol. 2000;391:137–144. doi: 10.1016/s0014-2999(99)00794-3. [DOI] [PubMed] [Google Scholar]
- Jourd'heuil D, Laroux FS, Miles AM, Wink DA, Grisham MB. Effect of superoxide dismutase on the stability of S-nitrosothiols. Arch Biochem Biophys. 1999;361:323–330. doi: 10.1006/abbi.1998.1010. [DOI] [PubMed] [Google Scholar]
- Kaposzta Z, Baskerville PA, Madge D, Fraser S, Martin JF, Markus HS. L-arginine and S-nitrosoglutathione reduce embolization in humans. Circulation. 2001;103:2371–2375. doi: 10.1161/01.cir.103.19.2371. [DOI] [PubMed] [Google Scholar]
- Kaposzta Z, Martin JF, Markus HS. Switching off embolization from symptomatic carotid plaque using S-nitrosoglutathione. Circulation. 2002;105:1480–1484. doi: 10.1161/01.cir.0000012347.47001.97. [DOI] [PubMed] [Google Scholar]
- Kashfi K, Rigas B. Molecular targets of nitric-oxide-donating aspirin in cancer. Biochem Soc Trans. 2005;33:701–704. doi: 10.1042/BST0330701. [DOI] [PubMed] [Google Scholar]
- Katsumi H, Nishikawa M, Yamashita F, Hashida M. Development of polyethylene glycol-conjugated poly-S-nitrosated serum albumin, a novel S-Nitrosothiol for prolonged delivery of nitric oxide in the blood circulation in vivo. J Pharmacol Exp Ther. 2005;314:1117–1124. doi: 10.1124/jpet.105.087429. [DOI] [PubMed] [Google Scholar]
- Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvao M, et al. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol. 1992;19:918–925. doi: 10.1016/0735-1097(92)90271-n. [DOI] [PubMed] [Google Scholar]
- Kaul S, Cercek B, Rengstrom J, Xu XP, Molloy MD, Dimayuga P, et al. Polymeric-based perivascular delivery of a nitric oxide donor inhibits intimal thickening after balloon denudation arterial injury: role of nuclear factor-kappaB. J Am Coll Cardiol. 2000;35:493–501. doi: 10.1016/s0735-1097(99)00543-4. [DOI] [PubMed] [Google Scholar]
- Kavdia M, Lewis RS. Nitric oxide delivery in stagnant systems via nitric oxide donors: a mathematical model. Chem Res Toxicol. 2003;16:7–14. doi: 10.1021/tx025528r. [DOI] [PubMed] [Google Scholar]
- Keeble JE, Moore PK. Pharmacology and potential therapeutic applications of nitric oxide-releasing non-steroidal anti-inflammatory and related nitric oxide-donating drugs. Br J Pharmacol. 2002;137:295–310. doi: 10.1038/sj.bjp.0704876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F, Greig IR, Newton DJ, Butler AR, Belch JJ. Skin blood flow after transdermal S-nitrosothio-acetylglucose. Lancet. 1997;350:410–411. doi: 10.1016/S0140-6736(05)64133-5. [DOI] [PubMed] [Google Scholar]
- Kleschyov AL, Oelze M, Daiber A, Huang Y, Mollnau H, Schulz E, et al. Does nitric oxide mediate the vasodilator activity of nitroglycerin. Circ Res. 2003;93:e104–e112. doi: 10.1161/01.RES.0000100067.62876.50. [DOI] [PubMed] [Google Scholar]
- Kojda G.Therapeutic importance of nitrovasodilators Nitric Oxide 2000Springer: Berlin Heidelberg; 365–384.In: Mayer B (ed) [Google Scholar]
- Kollau A, Hofer A, Russwurm M, Koesling D, Keung WM, Schmidt K, et al. Contribution of aldehyde dehydrogenase to mitochondrial bioactivation of nitroglycerin: evidence for the activation of purified soluble guanylate cyclase through direct formation of nitric oxide. Biochem J. 2005;385:769–777. doi: 10.1042/BJ20041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger MH, Santos KF, Shishido SM, Wanschel AC, Estrela HF, Santos L, et al. Antiatherogenic effects of S-nitroso-N-acetylcysteine in hypercholesterolemic LDL receptor knockout mice. Nitric Oxide. 2006;14:12–20. doi: 10.1016/j.niox.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Kurz MA, Lamping KG, Bates JN, Eastham CL, Marcus ML, Harrison DG. Mechanisms responsible for the heterogeneous coronary microvascular response to nitroglycerin. Circ Res. 1991;68:847–855. doi: 10.1161/01.res.68.3.847. [DOI] [PubMed] [Google Scholar]
- Lam CF, Caterina P, Filion P, Ilett KF, van Heerden PV. The safety of aerosolized diethylenetriamine nitric oxide adduct after single-dose administration to anesthetized piglets and multiple-dose administration to conscious rats. Toxicol Appl Pharmacol. 2003;190:65–71. doi: 10.1016/s0041-008x(03)00193-5. [DOI] [PubMed] [Google Scholar]
- Lam CF, van Heerden PV, Sviri S, Roberts BL, Ilett KF. The effects of inhalation of a novel nitric oxide donor, DETA/NO, in a patient with severe hypoxaemia due to acute respiratory distress syndrome. Anaesth Intensive Care. 2002;30:472–476. doi: 10.1177/0310057X0203000413. [DOI] [PubMed] [Google Scholar]
- Lamping KG, Bloom EN. Comparison of coronary microvascular response to nipradilol and nitroglycerin. Pharmacology. 1995;51:315–322. doi: 10.1159/000139341. [DOI] [PubMed] [Google Scholar]
- Langford EJ, Brown AS, Wainwright RJ, de Belder AJ, Thomas MR, Smith RE, et al. Inhibition of platelet activity by S-nitrosoglutathione during coronary angioplasty. Lancet. 1994;344:1458–1460. doi: 10.1016/s0140-6736(94)90287-9. [DOI] [PubMed] [Google Scholar]
- Lechi C, Andrioli G, Gaino S, Tommasoli R, Zuliani V, Ortolani R, et al. The antiplatelet effects of a new nitroderivative of acetylsalicylic acid – an in vitro study of inhibition on the early phase of platelet activation and on TXA2 production. Thromb Haemost. 1996;76:791–798. [PubMed] [Google Scholar]
- Lees C, Langford E, Brown AS, de Belder A, Pickles A, Martin JF, et al. The effects of S-nitrosoglutathione on platelet activation, hypertension, and uterine and fetal Doppler in severe preeclampsia. Obstet Gynecol. 1996;88:14–19. doi: 10.1016/0029-7844(96)00070-1. [DOI] [PubMed] [Google Scholar]
- Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- Loscalzo J, Smick D, Andon N, Cooke J. S-nitrosocaptopril. I. Molecular characterization and effects on the vasculature and on platelets. J Pharmacol Exp Ther. 1989;249:726–729. [PubMed] [Google Scholar]
- Loubani M, Galinanes M. Long-term administration of nicorandil abolishes ischemic and pharmacologic preconditioning of the human myocardium: role of mitochondrial adenosine triphosphate-dependent potassium channels. J Thorac Cardiovasc Surg. 2002;124:750–757. doi: 10.1067/mtc.2002.126037. [DOI] [PubMed] [Google Scholar]
- Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- Ma L, Wallace JL. Endothelial nitric oxide synthase modulates gastric ulcer healing in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G341–G346. doi: 10.1152/ajpgi.2000.279.2.G341. [DOI] [PubMed] [Google Scholar]
- Maalej N, Albrecht R, Loscalzo J, Folts JD. The potent platelet inhibitory effects of S-nitrosated albumin coating of artificial surfaces. J Am Coll Cardiol. 1999;33:1408–1414. doi: 10.1016/s0735-1097(98)00687-1. [DOI] [PubMed] [Google Scholar]
- Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, et al. Complexes of·NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J Med Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- Marks DS, Vita JA, Folts JD, Keaney JF, Jr, Welch GN, Loscalzo J. Inhibition of neointimal proliferation in rabbits after vascular injury by a single treatment with a protein adduct of nitric oxide. J Clin Invest. 1995;96:2630–2638. doi: 10.1172/JCI118328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M, Keeble J, Moore PK. Effect of a nitric oxide releasing derivative of paracetamol in a rat model of endotoxaemia. Br J Pharmacol. 2006;149:516–522. doi: 10.1038/sj.bjp.0706855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters KS, Leibovich SJ, Belem P, West JL, Poole-Warren LA. Effects of nitric oxide releasing poly (vinyl alcohol) hydrogel dressing on dermal wound healing in diabetic mice. Br J Surg. 2002;89:1594–1601. doi: 10.1046/j.1524-475x.2002.10503.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Takahashi M, Nakae I, Kinoshita M. Vasorelaxing effect of S-nitrosocaptopril on dog coronary arteries: no cross-tolerance with nitroglycerin. J Pharmacol Exp Ther. 1995;275:1247–1253. [PubMed] [Google Scholar]
- Matsuo H, Watanabe S, Segawa T, Yasuda S, Hirose T, Iwama M, et al. Evidence of pharmacologic preconditioning during PTCA by intravenous pretreatment with ATP-sensitive K+ channel opener nicorandil. Eur Heart J. 2003;24:1296–1303. doi: 10.1016/s0195-668x(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Megson IL, Webb DJ. Nitric oxide donor drugs: current status and future trends. Expert Opin Investig Drugs. 2002;11:587–601. doi: 10.1517/13543784.11.5.587. [DOI] [PubMed] [Google Scholar]
- Megson IL. Nitric oxide donor drugs. Drugs of the Future. 2000;25:701–715. [Google Scholar]
- Megson IL. Lipophilic S-nitrosothiols: a means of targeted delivery of nitric oxide to areas of endothelial injury. Drugs of the Future. 2002;27:777–781. [Google Scholar]
- Megson IL, Greig IR, Gray GA, Webb DJ, Butler AR. Prolonged effect of a novel S-nitrosated glyco-amino acid in endothelium-denuded rat femoral arteries: potential as a slow release nitric oxide donor drug. Br J Pharmacol. 1997;122:1617–1624. doi: 10.1038/sj.bjp.0701557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megson IL, Morton S, Greig IR, Mazzei FA, Field RA, Butler AR, et al. N-Substituted analogues of S-nitroso-N-acetyl-D, L-penicillamine: chemical stability and prolonged nitric oxide mediated vasodilatation in isolated rat femoral arteries. Br J Pharmacol. 1999;126:639–648. doi: 10.1038/sj.bjp.0702346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hanspal IS, Hadoke PW, Newby DE, Rossi AG, Webb DJ, et al. A novel S-nitrosothiol causes prolonged and selective inhibition of platelet adhesion at sites of vascular injury. Cardiovasc Res. 2003;57:853–860. doi: 10.1016/s0008-6363(02)00779-4. [DOI] [PubMed] [Google Scholar]
- Miller MR, Okubo K, Roseberry MJ, Webb DJ, Megson IL. Extracellular nitric oxide release mediates soluble guanylate cyclase-independent vasodilator action of spermine NONOate: comparison with other nitric oxide donors in isolated rat femoral arteries. J Cardiovasc Pharmacol. 2004;43:440–451. doi: 10.1097/00005344-200403000-00016. [DOI] [PubMed] [Google Scholar]
- Miller MR, Roseberry MJ, Mazzei FA, Butler AR, Webb DJ, Megson IL. Novel S-nitrosothiols do not engender vascular tolerance and remain effective in glyceryltrinitrate-tolerant rat femoral arteries. Eur J Pharmacol. 2000;408:335–343. doi: 10.1016/s0014-2999(00)00777-9. [DOI] [PubMed] [Google Scholar]
- Minuz P, Degan M, Gaino S, Meneguzz A, Zuliani V, Santonastaso CL, et al. NCX4016 (NO-Aspirin) has multiple inhibitory effects in LPS-stimulated human monocytes. Br J Pharmacol. 2001;134:905–911. doi: 10.1038/sj.bjp.0704326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy J, Martin JF, Baskerville PA, Fraser SC, Markus HS. S-nitrosoglutathione reduces the rate of embolization in humans. Circulation. 1998;98:1372–1375. doi: 10.1161/01.cir.98.14.1372. [DOI] [PubMed] [Google Scholar]
- Momi S, Emerson M, Paul W, Leone M, Mezzasoma AM, Del Soldato P, et al. Prevention of pulmonary thromboembolism by NCX 4016, a nitric oxide-releasing aspirin. Eur J Pharmacol. 2000;397:177–185. doi: 10.1016/s0014-2999(00)00223-5. [DOI] [PubMed] [Google Scholar]
- Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol. 1995;25:674–678. [PubMed] [Google Scholar]
- Morley D, Keefer LK. Nitric oxide/nucleophile complexes: a unique class of nitric oxide-based vasodilators. J Cardiovasc Pharmacol. 1993;22 Suppl 7:S3–S9. [PubMed] [Google Scholar]
- Morley D, Maragos CM, Zhang XY, Boignon M, Wink DA, Keefer LK. Mechanism of vascular relaxation induced by the nitric oxide (NO)/nucleophile complexes, a new class of NO-based vasodilators. J Cardiovasc Pharmacol. 1993;21:670–676. doi: 10.1097/00005344-199304000-00023. [DOI] [PubMed] [Google Scholar]
- Mowery KA, Schoenfisch MH, Saavedra JE, Keefer LK, Meyerhoff ME. Preparation and characterization of hydrophobic polymeric films that are thromboresistant via nitric oxide release. Biomaterials. 2000;21:9–21. doi: 10.1016/s0142-9612(99)00127-1. [DOI] [PubMed] [Google Scholar]
- Munzel T, Hink U, Yigit H, Macharzina R, Harrison DG, Mulsch A. Role of superoxide dismutase in in vivo and in vitro nitrate tolerance. Br J Pharmacol. 1999;127:1224–1230. doi: 10.1038/sj.bjp.0702622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T, Li H, Mollnau H, Hink U, Matheis E, Hartmann M, et al. Effects of long-term nitroglycerin treatment on endothelial nitric oxide synthase (NOS III) gene expression, NOS III-mediated superoxide production, and vascular NO bioavailability. Circ Res. 2000;86:E7–E12. doi: 10.1161/01.res.86.1.e7. [DOI] [PubMed] [Google Scholar]
- Munzel T, Sayegh H, Freeman BA, Tarpey MM, Harrison DG. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J Clin Invest. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Moss AJ, Brown MW, Kinoshita M, Kawai C. Long-term nitrate use may be deleterious in ischemic heart disease: a study using the databases from two large-scale postinfarction studies. Multicenter Myocardial Ischemia Research Group. Am Heart J. 1999;138:577–585. doi: 10.1016/s0002-8703(99)70163-8. [DOI] [PubMed] [Google Scholar]
- Napoli C, Ignarro LJ. Nitric oxide-releasing drugs. Annu Rev Pharmacol Toxicol. 2003;43:97–123. doi: 10.1146/annurev.pharmtox.43.100901.140226. [DOI] [PubMed] [Google Scholar]
- Napoli C, Aldini G, Wallace JL, de Nigris F, Maffei R, Abete P, et al. Efficacy and age-related effects of nitric oxide-releasing aspirin on experimental restenosis. Proc Natl Acad Sci USA. 2002;99:1689–1694. doi: 10.1073/pnas.022639399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, Cirino G, Del Soldato P, Sorrentino R, Sica V, Condorelli M, et al. Effects of nitric oxide-releasing aspirin versus aspirin on restenosis in hypercholesterolemic mice. Proc Natl Acad Sci USA. 2001;98:2860–2864. doi: 10.1073/pnas.041602898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KA, Boon NA, et al. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation. 1999;99:1411–1415. doi: 10.1161/01.cir.99.11.1411. [DOI] [PubMed] [Google Scholar]
- Nielsen VG. Nitric oxide decreases coagulation protein function in rabbits as assessed by thromboelastography. Anesth Analg. 2001;92:320–323. doi: 10.1097/00000539-200102000-00006. [DOI] [PubMed] [Google Scholar]
- Ongini E, Impagnatiello F, Bonazzi A, Guzzetta M, Govoni M, Monopoli A, et al. Nitric oxide (NO)-releasing statin derivatives, a class of drugs showing enhanced antiproliferative and antiinflammatory properties. Proc Natl Acad Sci USA. 2004;101:8497–8502. doi: 10.1073/pnas.0401996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta RM, Oldfield EH, Boock RJ. Reversal and prevention of cerebral vasospasm by intracarotid infusions of nitric oxide donors in a primate model of subarachnoid hemorrhage. J Neurosurg. 1997;87:746–751. doi: 10.3171/jns.1997.87.5.0746. [DOI] [PubMed] [Google Scholar]
- Pukrein G, Yancy CW. Bidil's impact. Nat Biotechnol. 2005;23:1343. doi: 10.1038/nbt1105-1343a. [DOI] [PubMed] [Google Scholar]
- Quintana A, Rodriguez JV, Scandizzi A, Guibert EE. Effect of S-nitrosoglutathione (GSNO) added to the University of Wisconsin solution (UW): I) Morphological alteration during cold preservation/reperfusion of rat liver. Int J Surg Investig. 2001;2:401–411. [PubMed] [Google Scholar]
- Rajaratnam R, Brieger DB, Hawkins R, Freedman SB.Attenuation of anti-ischemic efficacy during chronic therapy with nicorandil in patients with stable angina pectoris Am J Cardiol 1999831120–1124.A9 [DOI] [PubMed] [Google Scholar]
- Ramachandran N, Root P, Jiang XM, Hogg PJ, Mutus B. Mechanism of transfer of NO from extracellular S-nitrosothiols into the cytosol by cell-surface protein disulfide isomerase. Proc Natl Acad Sci USA. 2001;98:9539–9544. doi: 10.1073/pnas.171180998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay B, Radomski M, De Belder A, Martin JF, Lopez-Jaramillo P. Systemic effects of S-nitroso-glutathione in the human following intravenous infusion. Br J Clin Pharmacol. 1995;40:101–102. doi: 10.1111/j.1365-2125.1995.tb04545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhala P, Andoh T, Chiueh CC. Neuroprotective properties of nitric oxide and S-nitrosoglutathione. Toxicol Appl Pharmacol. 2005;207:91–95. doi: 10.1016/j.taap.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rossoni G, Berti M, Colonna VD, Bernareggi M, Del Soldato P, Berti F. Myocardial protection by the nitroderivative of aspirin, NCX 4016: in vitro and in vivo experiments in the rabbit. Ital Heart J. 2000;1:146–155. [PubMed] [Google Scholar]
- Rossoni G, Manfredi B, Colonna VD, Bernareggi M, Berti F. The nitroderivative of aspirin, NCX 4016, reduces infarct size caused by myocardial ischemia–reperfusion in the anesthetized rat. J Pharmacol Exp Ther. 2001;297:380–387. [PubMed] [Google Scholar]
- Ryan J, Cohen DJ. Are drug-eluting stents cost-effective? It depends on whom you ask. Circulation. 2006;114:1736–1743. doi: 10.1161/CIRCULATIONAHA.105.546010. [DOI] [PubMed] [Google Scholar]
- Saavedra JE, Billiar TR, Williams DL, Kim YM, Watkins SC, Keefer LK. Targeting nitric oxide (NO) delivery in vivo. Design of a liver-selective NO donor prodrug that blocks tumor necrosis factor-α-induced apoptosis and toxicity in the liver. J Med Chem. 1997;40:1947–1954. doi: 10.1021/jm9701031. [DOI] [PubMed] [Google Scholar]
- Saavedra JE, Booth MN, Hrabie JA, Davies KM, Keefer LK. Piperazine as a linker for incorporating the nitric oxide-releasing diazeniumdiolate group into other biomedically relevant functional molecules. J Org Chem. 1999;64:5124–5131. doi: 10.1021/jo9901539. [DOI] [PubMed] [Google Scholar]
- Saavedra JE, Mooradian DL, Mowery KA, Schoenfisch MH, Citro ML, Davies KM, et al. Conversion of a polysaccharide to nitric oxide-releasing form. Dual-mechanism anticoagulant activity of diazeniumdiolated heparin. Bioorg Med Chem Lett. 2000a;10:751–753. doi: 10.1016/s0960-894x(00)00086-x. [DOI] [PubMed] [Google Scholar]
- Saavedra JE, Shami PJ, Yi Wang L, Davies KM, Booth NB, Citro ML, et al. Esterase-sensitive nitric oxide donors of the diazeniumdiolate family: in vitro antileukemic activity. J Med Chem. 2000b;43:261–269. doi: 10.1021/jm9903850. [DOI] [PubMed] [Google Scholar]
- Saavedra JE, Southan GJ, Davies KM, Lundell A, Markou C, Hanson S, et al. Localising antithrombotic and vasodilatory activity with a novel, ultrafast nitric oxide donor. J Med Chem. 1996;39:4361–4365. doi: 10.1021/jm960616s. [DOI] [PubMed] [Google Scholar]
- Sage PR, de la Lande IS, Stafford I, Bennett CL, Phillipov G, Stubberfield J, et al. Nitroglycerin tolerance in human vessels: evidence for impaired nitroglycerin bioconversion. Circulation. 2000;102:2810–2815. doi: 10.1161/01.cir.102.23.2810. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Kaikita K, Miyamoto S, Kojima S, Sugiyama S, Yoshimura M, et al. Effects of nicorandil on endogenous fibrinolytic capacity in patients with coronary artery disease. Circ J. 2004;68:232–235. doi: 10.1253/circj.68.232. [DOI] [PubMed] [Google Scholar]
- Salas E, Langford EJ, Marrinan MT, Martin JF, Moncada S, de Belder AJ. S-nitrosoglutathione inhibits platelet activation and deposition in coronary artery saphenous vein grafts in vitro and in vivo. Heart. 1998;80:146–150. doi: 10.1136/hrt.80.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawatzky DA, Megson IL, Rossi AG. Sildenafil offers protection against NSAID-induced gastric injury. Br J Pharmacol. 2005;146:477–478. doi: 10.1038/sj.bjp.0706362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfisch MH, Mowery KA, Rader MV, Baliga N, Wahr JA, Meyerhoff ME. Improving the thromboresistivity of chemical sensors via nitric oxide release: fabrication and in vivo evaluation of NO-releasing oxygen-sensing catheters. Anal Chem. 2000;72:1119–1126. doi: 10.1021/ac991370c. [DOI] [PubMed] [Google Scholar]
- Schulz G, Stechmiller J. Wound healing and nitric oxide production: too little or too much may impair healing and cause chronic wounds. Int J Low Extrem Wounds. 2006;5:6–8. doi: 10.1177/1534734606286633. [DOI] [PubMed] [Google Scholar]
- Seabra AB, Fitzpatrick A, Paul J, De Oliveira MG, Weller R. Topically applied S-nitrosothiol-containing hydrogels as experimental and pharmacological nitric oxide donors in human skin. Br J Dermatol. 2004;151:977–983. doi: 10.1111/j.1365-2133.2004.06213.x. [DOI] [PubMed] [Google Scholar]
- Shabani M, Simmon M, Smith DJ, Dawood Al-Waili NS, Haq H. Transdermal delivery from nitric oxide-complexes (NONOates) FASEB J. 2001;15:A146. [Google Scholar]
- Shaffer JE, Han BJ, Chern WH, Lee FW. Lack of tolerance to a 24-hour infusion of S-nitroso N-acetylpenicillamine (SNAP) in conscious rabbits. J Pharmacol Exp Ther. 1992;260:286–293. [PubMed] [Google Scholar]
- Shaffer JE, Lee F, Thomson S, Han BJ, Cooke JP, Loscalzo J. The hemodynamic effects of S-nitrosocaptopril in anesthetized dogs. J Pharmacol Exp Ther. 1991;256:704–709. [PubMed] [Google Scholar]
- Shami PJ, Saavedra JE, Wang LY, Bonifant CL, Diwan BA, Singh SV, et al. JS-K, a glutathione/glutathione-S-transferase-activated nitric oxide donor of the diazeniumdiolate class with potent antineoplastic activity. Mol Cancer Therap. 2003;2:409–417. [PubMed] [Google Scholar]
- Sica DA. BiDil (isosorbide dinitrate and hydralazine hydrochloride) Congest Heart Fail. 2006;12:110–111. doi: 10.1111/j.1527-5299.2006.04960.x. [DOI] [PubMed] [Google Scholar]
- Simpson D, Wellington K. Nicorandil: a review of its use in the management of stable angina pectoris, including high-risk patients. Drugs. 2004;64:1941–1955. doi: 10.2165/00003495-200464170-00012. [DOI] [PubMed] [Google Scholar]
- Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Ann Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- Sogo N, Campanella C, Webb DJ, Megson IL. S-nitrosothiols cause prolonged, nitric oxide-mediated relaxation in human saphenous vein and internal mammary artery: therapeutic potential in bypass surgery. Br J Pharmacol. 2000a;131:1236–1244. doi: 10.1038/sj.bjp.0703700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo N, Magid KS, Shaw CA, Webb DJ, Megson IL. Inhibition of human platelet aggregation by nitric oxide donor drugs: relative contribution of cGMP-independent mechanisms. Biochem Biophys Res Commun. 2000b;279:412–419. doi: 10.1006/bbrc.2000.3976. [DOI] [PubMed] [Google Scholar]
- Sogo N, Wilkinson IB, MacCallum H, Khan SQ, Strachan FE, Newby DE, et al. A novel S-nitrosothiol (RIG200) causes prolonged relaxation in dorsal hand veins with damaged endothelium. Clin Pharmacol Ther. 2000c;68:75–81. doi: 10.1067/mcp.2000.107049. [DOI] [PubMed] [Google Scholar]
- Sorba G, Galli U, Cena C, Fruttero R, Gasco A, Morini G, et al. A new furoxan NO-donor rabeprazole derivative and related compounds. Chembiochem. 2003;4:899–903. doi: 10.1002/cbic.200300617. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Simon DI, Jaraki O, Osborne JA, Francis S, Mullins M, et al. S-nitrosylation of tissue-type plasminogen activator confers vasodilatory and antiplatelet properties on the enzyme. Proc Natl Acad Sci USA. 1992;89:8087–8091. doi: 10.1073/pnas.89.17.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydow K, Daiber A, Oelze M, Chen Z, August M, Wendt M, et al. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J Clin Invest. 2004;113:482–489. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallet D, Del Soldato P, Oudart N, Burgaud JL. NO-steroids: potent anti-inflammatory drugs with bronchodilating activity in vitro. Biochem Biophys Res Commun. 2002;290:125–130. doi: 10.1006/bbrc.2001.6192. [DOI] [PubMed] [Google Scholar]
- Talukdar A, Wang PG.N-Nitroso compounds Nitric Oxide Donors 2005Wiley-VCH: Weinheim; 55–89.In: Wang PG, Cai TB, Taniguchi N (eds) [Google Scholar]
- Tang X, Xian M, Trikha M, Honn KV, Wang PG. Synthesis of peptide-dizeniumdiolate conjugates: towards enzyme activated antitumor agents. Tetrahedron Lett. 2001;42:2625–2629. [Google Scholar]
- Taylor EL, Megson IL, Haslett C, Rossi AG. Nitric oxide: a key regulator of myeloid inflammatory cell apoptosis. Cell Death Diff. 2003;10:418–430. doi: 10.1038/sj.cdd.4401152. [DOI] [PubMed] [Google Scholar]
- Thakur NK, Hayashi T, Sumi D, Kano H, Matsui-Hirai H, Tsunekawa T, et al. Anti-atherosclerotic effect of beta-blocker with nitric oxide-releasing action on the severe atherosclerosis. J Cardiovasc Pharmacol. 2002;39:298–309. doi: 10.1097/00005344-200202000-00017. [DOI] [PubMed] [Google Scholar]
- Thatcher GR, Nicolescu AC, Bennett BM, Toader V. Nitrates and NO release: contemporary aspects in biological and medicinal chemistry. Free Radic Biol Med. 2004;37:1122–1143. doi: 10.1016/j.freeradbiomed.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Trujillo M, Alvarez MN, Peluffo G, Freeman BA, Radi R. Xanthine oxidase-mediated decomposition of S-nitrosothiols. J Biol Chem. 1998;273:7828–7834. doi: 10.1074/jbc.273.14.7828. [DOI] [PubMed] [Google Scholar]
- Turnbull CM, Cena C, Fruttero R, Gasco A, Rossi AG, Megson IL. Mechanism of action of novel NO-releasing furoxan derivatives of aspirin in human platelets. Br J Pharmacol. 2006a;148:517–526. doi: 10.1038/sj.bjp.0706743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull CM, Rossi AG, Megson IL. Therapeutic effects of nitric oxide-aspirin hybrid drugs. Exp Opin Therap Targets. 2006b;10:911–922. doi: 10.1517/14728222.10.6.911. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Nakamura M, Shimizu S, Shirasawa Y, Fujii M. Vasoactive and beta-adrenoceptor blocking properties of 3, 4-dihydro-8-(2-hydroxy-3-isopropylamino) propoxy-3-nitroxy-2H-1-benzopyran (K-351), a new antihypertensive agent. Arch Int Pharmacodyn Ther. 1983;262:132–149. [PubMed] [Google Scholar]
- Vanderford PA, Wong J, Chang R, Keefer LK, Soifer SJ, Fineman JR. Diethylamine/nitric oxide (NO) adduct, an NO donor, produces potent pulmonary and systemic vasodilation in intact newborn lambs. J Cardiovasc Pharmacol. 1994;23:113–119. doi: 10.1097/00005344-199401000-00016. [DOI] [PubMed] [Google Scholar]
- Velazquez C, Praveen Rao PN, Knaus EE. Novel nonsteroidal antiinflammatory drugs possessing a nitric oxide donor diazen-1-ium-1, 2-diolate moiety: design, synthesis, biological evaluation, and nitric oxide release studies. J Med Chem. 2005;48:4061–4067. doi: 10.1021/jm050211k. [DOI] [PubMed] [Google Scholar]
- Vilahur G, Baldellou MI, Segales E, Salas E, Badimon L. Inhibition of thrombosis by a novel platelet selective S-nitrosothiol compound without hemodynamic side effects. Cardiovasc Res. 2004;61:806–816. doi: 10.1016/j.cardiores.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Wainwright CL, Miller AM, Work LM, Del Soldato P. NCX4016 (NO-aspirin) reduces infarct size and suppresses arrhythmias following myocardial ischaemia/reperfusion in pigs. Br J Pharmacol. 2002;135:1882–1888. doi: 10.1038/sj.bjp.0704646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, Del Soldato P. The therapeutic potential of NO-NSAIDs. Fundam Clin Pharmacol. 2003;17:11–20. doi: 10.1046/j.1472-8206.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Ignarro LJ, Fiorucci S. Potential cardioprotective actions of no-releasing aspirin. Nat Rev Drug Discov. 2002;1:375–382. doi: 10.1038/nrd794. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Muscara MN, McKnight W, Dicay M, Del Soldato P, Cirino G. In vivo antithrombotic effects of a nitric oxide-releasing aspirin derivative, NCX-4016. Thromb Res. 1999a;93:43–50. doi: 10.1016/s0049-3848(98)00134-0. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Vergnolle N, Muscara MN, Asfaha S, Chapman K, McKnight W, et al. Enhanced anti-inflammatory effects of a nitric oxide-releasing derivative of mesalamine in rats. Gastroenterology. 1999b;117:557–566. doi: 10.1016/s0016-5085(99)70448-8. [DOI] [PubMed] [Google Scholar]
- Wheatley PS, Butler AR, Crane MS, Fox S, Xiao B, Rossi AG, et al. NO-releasing zeolites and their antithrombotic properties. J Am Chem Soc. 2006;128:502–509. doi: 10.1021/ja0503579. [DOI] [PubMed] [Google Scholar]
- Williams DLH. The Chemistry of S-nitrisothiols. Acc Chem Res. 1999;32:869–874. [Google Scholar]
- Winbury MM, Howe BB, Hefner MA. Effect of nitrates and other coronary dilators on large and small coronary vessels: an hypothesis for the mechanism of action of nitrates. J Pharmacol Exp Ther. 1969;168:70–95. [PubMed] [Google Scholar]
- Wu WP, Hao JX, Ongini E, Impagnatiello F, Presotto C, Wiesenfeld-Hallin Z, et al. A nitric oxide (NO)-releasing derivative of gabapentin, NCX 8001, alleviates neuropathic pain-like behavior after spinal cord and peripheral nerve injury. Br J Pharmacol. 2004;141:65–74. doi: 10.1038/sj.bjp.0705596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Tang X, Xian M, Wang PG. Glycosylated diazeniumdiolates: a novel class of enzyme-activated nitric oxide donors. Tetrahedron Let. 2001;42:3779–3782. [Google Scholar]
- Yamamoto T, Bing RJ. Nitric oxide donors. Proc Soc Exp Biol Med. 2000;225:200–206. doi: 10.1046/j.1525-1373.2000.22525.x. [DOI] [PubMed] [Google Scholar]
- Yin ZL, Dusting GJ. A nitric oxide donor (spermine-NONOate) prevents the formation of neointima in rabbit carotid artery. Clin Exp Pharmacol Physiol. 1997;24:436–438. doi: 10.1111/j.1440-1681.1997.tb01218.x. [DOI] [PubMed] [Google Scholar]
- Young DV, Cochran ED, Dhawan V, Earl RA, Ellis JL, Garvey DS, et al. A comparison of the cyclooxygenase inhibitor-NO donors (CINOD), NMI-1182 and AZD3582, using in vitro biochemical and pharmacological methods. Biochem Pharmacol. 2005;70:1343–1351. doi: 10.1016/j.bcp.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Yurtseven N, Karaca P, Kaplan M, Ozkul V, Tuygun AK, Aksoy T, et al. Effect of nitroglycerin inhalation on patients with pulmonary hypertension undergoing mitral valve replacement surgery. Anesthesiology. 2003;99:855–858. doi: 10.1097/00000542-200310000-00017. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Collins R, MacMahon S, Peto R. Effect of intravenous nitrates on mortality in acute myocardial infarction: an overview of the randomised trials. Lancet. 1988;1:1088–1092. doi: 10.1016/s0140-6736(88)91906-x. [DOI] [PubMed] [Google Scholar]
- Zai A, Rudd MA, Scribner AW, Loscalzo J. Cell-surface protein disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide. J Clin Invest. 1999;103:393–399. doi: 10.1172/JCI4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, de la Lande IS, Stafford I, Horowitz JD. S-Nitrosothiol modulation of tolerance to glyceryl trinitrate in bovine isolated coronary artery. Eur J Pharmacol. 1994;252:299–304. doi: 10.1016/0014-2999(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen Z, Cobb FR, Stamler JS. Role of mitochondrial aldehyde dehydrogenase in nitroglycerin-induced vasodilation of coronary and systemic vessels: an intact canine model. Circulation. 2004;110:750–755. doi: 10.1161/01.CIR.0000138105.17864.6B. [DOI] [PubMed] [Google Scholar]