Abstract
Background and purpose:
Our goal was to elucidate mechanisms of the inhibitory effect of rosuvastatin on the accumulation of plaque oxidized low density lipoproteins (oxLDL) and on plaque volume, without lowering cholesterol, in mice with combined leptin and LDL-receptor deficiency (DKO).
Experimental approach:
Twelve-week old DKO mice were treated with rosuvastatin (10 mg kg−1 day−1, s.c.) or placebo or no treatment for 12 weeks. The effect on blood variables, aortic plaque volume and composition and gene expression in the aorta and in THP-1 cells was assessed.
Key results:
Rosuvastatin lowered free fatty acids (FFA), triglycerides, and increased insulin sensitivity, without affecting cholesterol. Rosuvastatin lowered the plaque volume, inhibited macrophage, lipid and oxLDL accumulation, and decreased the oxLDL-to-LDL ratio of plaques in the aortic arch. It increased superoxide dismutase 1 (SOD1), CD36, LXR-α, ABCA-1 and PPAR-γ RNA expression in aortic extracts. SOD1 was the strongest inverse correlate of oxLDL. In THP-1 macrophages and foam cells, expression of SOD1 was lower than in THP-1 monocytes. Rosuvastatin restored expression of SOD1 in THP-1 macrophages and foam cells.
Conclusions and Implications:
Rosuvastatin restored SOD1 expression in THP-1 macrophages and foam cells in vitro and in the aorta of DKO mice. The latter was associated with less oxLDL accumulation within atherosclerotic plaques and inhibition of plaque progression. This effect was obtained at a dose not affecting cholesterol levels but improving insulin sensitivity. SOD1 is a potentially important mediator of the prevention of oxLDL accumulation within atherosclerotic plaques.
Keywords: atherosclerosis, cholesterol, lipoproteins, metabolic syndrome, obesity, superoxide dismutase
Introduction
Cardiovascular disease remains the leading cause of mortality in Western societies. Several risk factors predispose to cardiovascular disease including components of the metabolic syndrome: obesity, insulin resistance and diabetes, dyslipidemia and hypertension. Statin therapy results in significant cardiovascular disease risk reduction in patients with the metabolic syndrome (Lundbye and Thompson, 2005). Although the lowering of serum lipids, particularly low-density lipoproteins (LDL), plays an important role in this cardioprotective effect, other mechanisms are also likely to be involved. It has been shown in man that statins reduce insulin resistance (Paniagua et al., 2002; Costa et al., 2003; Sonmez et al., 2003; Guclu et al., 2004), a well-known risk factor for cardiovascular disease. However, how this reduction in insulin resistance contributes to the reduction in risk for cardiovascular disease is unclear. Recent studies have shown that statins also have important additional anti-inflammatory effects, which are independent of lipid lowering (Kleemann et al., 2003). These beneficial effects on vascular cells (Takemoto and Liao, 2001; Schonbeck and Libby, 2004) are mediated by peroxisome proliferator–activated receptors (PPARs) (Inoue et al., 2000; Grip et al., 2002). In human carotid plaques, statins have been shown to decrease oxidized LDL (oxLDL) and macrophages (Rosenson, 2004). The underlying mechanism is, however, not fully understood.
Removal of oxLDL from vascular macrophages is mediated through the reverse cholesterol transport pathway. PPAR-γ and liver X receptor α (LXR-α) link the pathway of oxLDL uptake via fatty acid translocase (CD36) to a pathway of cholesterol and phospholipid efflux via the ATP-binding cassette transporter A1 (ABCA-1). This enhances the ability of the macrophages to remove oxLDL from the vessel wall (Chawla et al., 2001). It has been shown that statins can induce PPAR-γ and LXR-α expression in macrophages in vitro and thereby increase cholesterol efflux via ABCA-1 (Argmann et al., 2005). However, the in vivo effects are less clear.
Oxidative modification of the LDL particles in the vessel wall plays a critical role in the development of atherosclerosis. As inflammation is closely linked to the production of reactive-oxygen species (ROS), the observed anti-inflammatory effects of statins may also relate to their ability to block the production and/or activity of ROS (Rosenson, 2004). It has previously been shown, in vitro and in vivo, that statins inhibit lipid and lipoprotein oxidation, thereby inhibiting ROS formation or blunting the damaging effects of these radicals (Wagner et al., 2000; Parker et al., 2003; Stoll et al., 2005).
An important antioxidant enzyme in vascular walls is superoxide dismutase (SOD). The main function of SOD is to scavenge O2− radicals (Faraci and Didion, 2004), thus preventing the oxidation of biological molecules (like LDL) (Fang et al., 1998). Two isoforms of SOD are localized within specific cellular compartments: copper-zinc SOD (SOD1) predominately within the cytosol and nucleus; and manganese SOD (SOD2) in the mitochondrial matrix. The third isoform (extracellular SOD or SOD3) is secreted and found primarily bound to heparin sulfate proteoglycan on cell surfaces. In blood vessels, SOD1 accounts for 50–80% of total SOD activity (Didion et al., 2002; Faraci and Didion, 2004). Mice deficient in SOD1 show increased superoxide and vascular dysfunction (Didion et al., 2002); overexpression of SOD1 in mice lacking apolipoprotein E resulted in the retardation of atherosclerosis (Fang et al., 1998). Furthermore, it has been shown that SOD1 is an important mediator of post ischaemic injury in the heart and that increasing intracellular SOD1 protects the heart from this injury (Wang et al., 1998; Tanaka et al., 2004; Fukushima et al., 2006). It has been suggested that SOD1 may play a crucial role in the antiatherogenic effects of statins (Umeji et al., 2006). To the best of our knowledge the effect of statins on SOD1 expression in the arterial wall in vivo, and its relation to the deposition of oxLDL have not yet been investigated. Because we have recently showed that SOD1 is the strongest inverse correlate of oxLDL in macrophages isolated from coronary plaques from hypercholesterolemic pigs (Holvoet et al., 2006), we have now focused on SOD1.
To investigate further the relationship between SOD1 and oxLDL, we selected mice with combined leptin and LDL-receptor deficiency (double knockout (DKO) mice) because they exhibit most of the components of the metabolic syndrome, which are associated with increased oxidative stress, accelerated atherosclerosis and impaired cardiovascular function (Mertens et al., 2003; Verreth et al., 2004, 2006; Mackness et al., 2006).
Methods
Breeding and genotyping
Homozygous LDL-receptor–knockout mice (LDLR(–/–)), heterozygous ob/+, and C57BL6 mice were purchased from Jackson Laboratory, Bar Harbor, ME, USA. LDLR(–/–) mice were backcrossed into a C57BL6 background to the 10th generation. To obtain DKO mice with combined leptin deficiency (ob/ob) and LDL-receptor deficiency, LDLR(–/–) mice were crossed with ob/+ mice as described previously (Mertens et al., 2003). All offspring were genotyped by polymerase chain reaction (PCR) techniques (Hasty et al., 2001). All mice were housed at 22°C on a fixed 12/12h light/dark cycle and were fed standard chow containing 4% fat (Pavan Service). Rosuvastatin (AstraZeneca, Macclesfield, UK), dissolved in physiological saline, was injected subcutaneously at a dosage of 10 mg kg−1 day−1 in 13 mice for 12 weeks starting at the age of 12 weeks. Placebo mice (n=11) were treated with vehicle only. We also included a control group of control DKO mice that did not receive any treatment (n=18). Control and placebo- and statin-treated DKO mice were compared with age- and gender-matched lean C57BL6 mice (n=10). Experimental procedures in animals were performed in accordance with protocols approved by the Institutional Animal Care and Research Advisory Committee.
Biochemical analyses
Blood from conscious mice was collected by tail bleeding into ethylenediaminetetraacetic acid tubes after an overnight fast. Plasma was obtained by centrifugation. Total cholesterol and triglycerides were measured with standard enzymatic assays (Boehringer Mannheim, Vilvoorde, Belgium). High-sensitivity lipoprotein profiling was performed on fractions separated with the LipoSEARCH system by Skylight Biotech Inc. (Tokyo, Japan) (Usui et al., 2002). Free-fatty acids (FFA) were measured with a FFA-Half Microtest kit (Roche Applied Science), glucose with glucometer (Menarini Diagnostics, Zaventen, Belgium) and plasma insulin with a mouse insulin enzyme-linked immunosorbent assay (Mercodia, Uppsala, Sweden). Insulin resistance was calculated by a homeostasis model assessment (HOMA)=fasting insulin (mU l−1) × fasting blood glucose (mmol l−1)/22.5. To determine glucose tolerance, glucose was measured in samples obtained by tail bleeding before and 15, 30, 60, 120 and 240 min after intraperitoneal glucose administration (20% glucose solution; 2 g kg−1) (Ludwig et al., 2001; Mertens et al., 2003; Verreth et al., 2004, 2006). The titers of immunoglobulin (Ig) autoantibody against malondialdehyde (MDA)-modified LDL were determined in individual plasma samples (1:500 dilution). The amount of Ig bound to the MDA-LDL antigen was detected with alkaline phosphatase–labelled anti-mouse IgG. Data are expressed as relative absorbance units (Tsimikas et al., 2001; Mertens et al., 2003).
Atherosclerosis
Because blood and metabolic parameters were comparable in control and placebo-treated DKO mice, both groups were combined and referred to as control DKO mice when determining plaque volume and composition. The extent of atherosclerosis was determined by analysis of histological cross-sections from the aortic root of 24-week old control (n=25) and statin-treated (n=10) DKO mice. Approximately 10–7-μm frozen sections per staining per animal were analyzed morphometrically and immunohistochemically. Lipids were stained with oil red O, LDL with AbL8A2 and oxLDL with mAb4E6 (Holvoet et al., 1998), smooth muscle cells (SMCs) with an α-actin-specific antibody (Dako), macrophages with an antibody against mouse Mac-3 antigen (Pharmingen, Hamburg, Germany), and SOD1 with a rabbit polyclonal anti-mouse SOD1 antibody (Santa Cruz, Heidelberg, Germany). A color intensity threshold mask for immunoassaying was defined to detect the red color by sampling and the same threshold was applied to all specimens. Analysis of positive-immunoassayed sections was carried out, without knowledge of treatments, using the Quantimet600 image analyzer (Leica, Brussels, Belgium) (Mertens et al., 2003; Verreth et al., 2004, 2006; Mackness et al., 2006). The positively immunostained area was expressed as a percentage of the total plaque area.
Real-time RT-PCR analysis
Real-time reverse transcription (RT)-PCR was used to determine the mRNA expression of PPAR-α, PPAR-γ, LXR-α, SOD1, CD36 and ABCA-1 in the aorta. Total RNA was extracted with the Trizol reagent (InVitrogen, Merelbeke, Belgium) and purified and treated with Rnase-free DNase on an RNeasy kit column (Qiagen, Antwerpen, Belgium). First-strand cDNA was generated from total RNA by RT, with random primers from Takara and Superscript III reverse transcriptase (InVitrogen). Quantitative real-time PCR was performed using SybrGreen master mix according to the supplier protocols (Applied Biosystems, Lennik, Belgium). Oligonucleotides (InVitrogen) used as forward primer (F) and reverse primer (R) were: for mouse PPAR-α: F: 5′-TCAGGGTACCACTACGG AGTTCA-3′; R: 5′-CCGAATAGTTCGCCGAAAGA-3′; for mouse PPAR-γ: F: 5′-GCAGCTACTGCATGTGATCAAGA-3′; R: 5″-GTCAGCGGGTGGGACTTTC-3′; for mouse LXR-α: F: 5′-GGAGTGTCGACTTCGCAAATG-3′; R: 5′-TCAAGCGGATCTG TTCTTCTGA-3′; for mouse CD36: F: 5′-CTCGGACATTGAG ATTCTTTTCCT-3′; R: 5′-GTCGATTTCAGATCCGAACACA-3′; for mouse ABCA-1: F: 5′-ACTTAGGGCACAATTCCACAAGA3′; R: 5′-CTCCTGTGGTGTTTCTGGATGA-3′; for mouse SOD1: F: 5′-GGGATTGCGCAGTAAACATTC-3′; R: 5′-AATGG TTTGAGGGTAGCAGATGA-3′; and for mouse β-actin (housekeeping gene), F: 5′-ACGGCCAGGTCATCACTATTG-3′; R: 5′-CACAGGATTCCATACCCAAGAAG-3′. The level of mRNA expression was calculated using the threshold cycle (Ct) value, that is the number of PCR cycles at which the fluorescent signal during the PCR reached a fixed threshold. For each sample, the Ct, both for the gene of interest and for β-actin, was determined to calculate ΔCt,sample (Ct,target gene−Ct,housekeeping gene), thus normalizing the data and correcting for differences in amount and/or quality between the different RNA samples. The expression levels were related to an external calibrator consisting of aortic tissue from C57BL6 control mice. Subsequently, ΔΔCt (ΔCt,sample−ΔCt,calibrator) was determined, and the relative expression levels were calculated from 2−ΔΔCt according to the manufacturer's instructions (Applied Biosystems). mRNA expression levels were expressed as a percentage of control (van Eck et al., 2003; Verreth et al., 2004, 2006).
Western blotting of SOD1
For the analysis of mouse SOD1 protein, total protein was extracted from aortas and concentrated on Amicon concentrators (between 10 and 50 kDa). Seven micrograms of the protein was separated on 15% sodium dodecyl sulfate gels, transferred onto nitrocellulose membranes, and stained with a rabbit polyclonal anti-mouse SOD1 antibody at a 1:200 dilution (Santa Cruz). The blots were incubated with horseradish peroxidase-coupled secondary antibody and developed with color development reagent. They were analyzed with the ‘Quantity One' analysis software (Bio-Rad, Nazareth, Belgium).
Gene expression in THP-1 cells
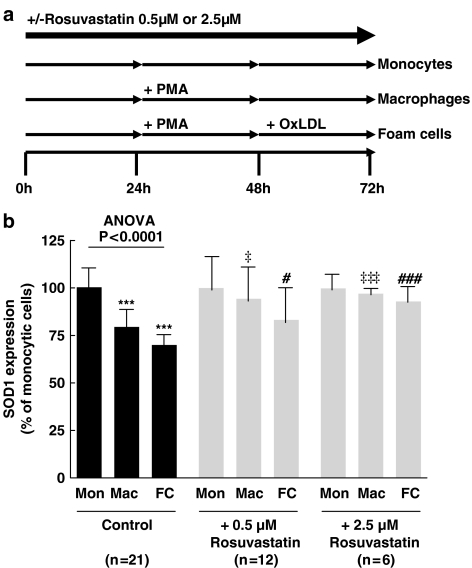
Figure 4a shows the experimental setup of the THP-1 experiments. THP-1 monocytic cells were maintained in Roswell Park Memorial Institute 1640 medium (GIBCO, Invitrogen, Merelbeke, Belgium) containing 10% fetal bovine serum (GIBCO, Invitrogen) and 5 μg ml−1 gentamicin (GIBCO, Invitrogen) at 37°C in 5% CO2. A total of 2 × 106 cells were cultured at a density of 1 × 106 cells ml−1 and seeded in 6-well plates for 24 h in the absence or presence of rosuvastatin at a final concentration of 0.5 or 2.5 μM, for the duration of the experiment. Cells were then cultured in the absence or presence of PMA (final concentration 10−6 M (Fluka) to induce THP-1 macrophage differentiation for 24 h. Thereafter cells were incubated for 24 h in serum-free medium containing 1 mg ml−1 HSA (Sigma, Bornem, Belgium), in the presence or absence of 50 μg ml−1 ox-LDL (N=6–21). The uptake of ox-LDL by THP-1 macrophages resulted in foam cell formation evidenced by the accumulation of numerous lipid droplets. Total RNA was extracted from THP-1 cells using RNeasy Mini kit (Qiagen). Quantitative real-time PCR analysis of SOD1 was performed as described above. Oligonucleotides (Invitrogen) used as forward (F) and reverse (R) primers were the following. For human β-actin, F: 5′-GGACCTGACCGACTACCTCATG-3′; R: 5′-CGACGTAGCAGAGCTTCTCCTT-3′; for human SOD1: F: 5′-TTGGGCAAAGGTGGAAATGA-3′; R: 5′-CACCACAAGCC AAACGACTTC-3′.
Figure 4.
(a) Experimental protocol for THP-1 studies. THP-1 monocytic cells were cultured in the absence or presence of rosuvastatin at a final concentration of 0.5 or 2.5 μM, for the duration of the experiment. Cells were then cultured in the absence or presence of PMA (final concentration 10−6 M) to induce THP-1 macrophage differentiation for 24 h. Thereafter cells were incubated for 24 h in serum-free medium in the presence or absence of 50 μg ml−1 ox-LDL. (b) Effect of rosuvastatin (0.5 or 2.5 μM on SOD1 RNA expression in THP-1 monocytes, macrophages and foam cells. Expression is related to monocytes with the same treatment. ***P<0.001 compared to control monocytes; ‡P<0.05 and ‡‡‡P<0.001 compared to control macrophages; #P<0.05 and ###P<0.001 compared to control foam cells (Mon=monocytes, Mac=macrophages, FC=foam cells).
Statistical analysis
Groups were compared by means of the Kruskal–Wallis test followed by Dunn's multiple-comparisons test or by Mann–Whitney test (Graph Pad Prism version 3.02). Regression analysis was performed with the Statistical Package for the Social Sciences (SPSS for Windows, release 10.0.5; Chicago, IL, USA). A probability value of P<0.05 was considered statistically significant.
Results
Weight and blood analysis
Body weight, plasma total cholesterol, triglycerides and FFA, were significantly higher in the control DKO mice compared with the same C57BL6 genetic background lean mice. Glucose and insulin levels, and thus the HOMA index were similarly elevated (Table 1). As expected, placebo treatment had no effect on any of these variables (Table 1). Rosuvastatin had no effect on weight, cholesterol levels or lipoprotein distribution. However, it reduced triglyceride and FFA levels, and decreased glucose and insulin resulting in a decrease in HOMA and in a partial normalization of glucose tolerance as measured by the area under the curve in an intraperitoneal glucose tolerance test (Table 1). Rosuvastatin had no effect on the titer of circulating antibodies against MDA-modified LDL (8.0±1.2 in control vs 8.3±3.3 in rosuvastatin treated mice, n=8 for both).
Table 1.
Blood and metabolic parameters
| Variable | C57BL6 (n=10) | DKO no treatment (n=18) | DKO placebo (n=11) | DKO rosuvastatin (n=13) |
|---|---|---|---|---|
| Weight (g) | 26±4 | 59±8‡‡‡ | 61±4‡‡‡ | 60±5‡‡‡ |
| Total cholesterol (mM) | 2.0±0.5 | 15.3±5.4‡‡‡ | 14.5±2.3‡‡‡ | 14.2±6.5‡‡‡ |
| Triglycerides (mM) | 0.26±0.06 | 4.5±2.2‡‡‡ | 5.4±1.5‡‡‡ | 2.9±1.8‡‡‡, #, ** |
| Free-fatty acids (mM) | 0.093±0.020 | 0.448±0.228‡‡‡ | 0.574±0.158‡‡‡ | 0.172±0.082‡, ###, *** |
| Glucose (mM) | 4.3±0.83 | 9.1±1.6‡‡‡ | 9.3±1.1‡‡‡ | 6.9±1.3‡‡‡, ###, *** |
| Insulin (mU l−1) | 130±10 | 4308±1554‡‡‡ | 4362±1789‡‡‡ | 2789±1507‡‡‡, #, * |
| HOMA | 25±4 | 1762±766‡‡‡ | 1812±735‡‡‡ (N=5) | 836±461‡‡‡, ###, ** |
| AUC of GTT (× 103) | 28±5.6 | 87±9.8‡‡‡ | 91±8.8‡‡‡ | 77±6.0‡‡‡, #, * |
Abbreviations: AUC, area under curve; DKO, double knockout; HOMA, homeostasis model assessment.
Data shown in the table are mean±s.d. HOMA=fasting insulin (mU l−1) × fasting blood glucose (mM)/22.5. AUC of GTT is the area under the curve in the glucose tolerance test. For all parameters ANOVA was P<0.0001. ‡P<0.05 and ‡‡‡P<0.001 compared to C57BL6; #P<0.05 and ###P<0.001 compared to non-treated DKO mice; *P<0.05, **P<0.01 and ***P<0.001 compared to placebo DKO mice.
Atherosclerosis
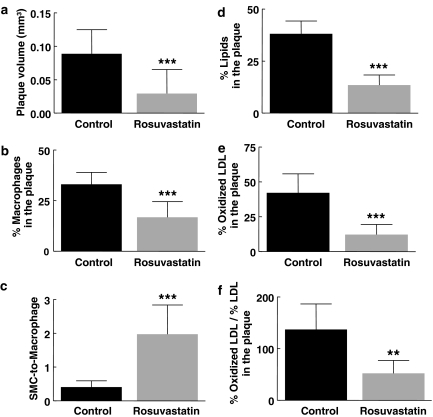
Rosuvastatin decreased plaque volume by 67% (Figure 1a) and changed plaque composition. Together with the 49% decrease in macrophage area (Figures 1b and 3), the twofold increase in SMC area (27±6 vs 13±6%; P<0.0001) resulted in a 4.8-fold increase of the SMC-to-macrophage ratio (Figure 1c). Rosuvastatin decreased plaque lipids (−65%) (Figure 1d) and oxLDL (−71%) (Figure 1e). As there was no statistical difference in LDL staining (39±9% in control vs 29±14% in rosuvastatin-treated mice, n=6 for both) the large reduction in the oxLDL-to-LDL ratio (62%) is attributable almost entirely to a reduction in oxLDL burden in the plaque (Figure 1f). Using Fab2 fragments to reduce interaction with Fc receptors on macrophages that contain most of the oxLDL, we found a comparable 70% decrease in plaque oxLDL in statin-treated (n=9; 12.1±5.5%) compared to control mice (n=13; 3.6±2.9%; P<0.001).
Figure 1.
Plaque volume (a), macrophage area (b), SMC-to-macrophage ratio (c), lipid area (d), oxLDL area (e) and oxLDL-to-LDL ratio, in rosuvastatin treated (n=10) and control (n=25) DKO mice at 24 weeks (n=6 in both groups for oxLDL-to-LDL ratio). Macrophage, lipid and oxLDL areas are presented as a percentage of plaque area. **P<0.01 and ***P<0.001 compared to control DKO mice.
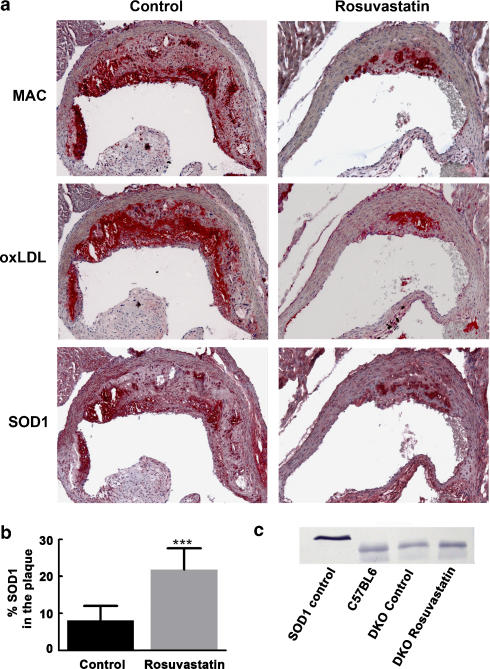
Figure 3.
(a) Representative staining for macrophages (MAC), ox-LDL, and SOD1 in control and rosuvastatin-treated DKO mice showing co-localization of these three components (red=positive staining). (b) SOD1 area (as percentage of plaque area) in control and rosuvastatin treated DKO mice at 24 weeks. (c) Detection of SOD1 by Western blotting of human SOD1 control (23 kDa) and in extracts from the aorta of lean C57BL6 mice and in control and rosuvastatin treated DKO mice (19 kDa). ***P<0.001 compared to control DKO mice.
Gene expression in the aorta
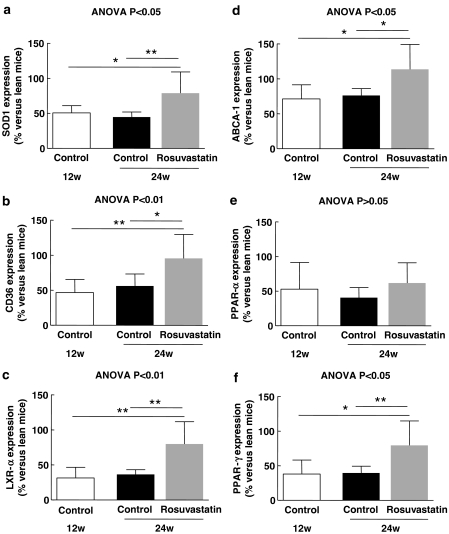
At 12 weeks, before atherosclerotic lesions were prominent, the expression of SOD1, CD36, LXR-α, ABCA-1, PPAR-α and PPAR-γ was lower in control DKO mice compared with that in lean C57BL6 mice (Figure 2). Placebo treatment had no effect on the expression of these genes. Rosuvastatin increased the expression of SOD1, CD36, and LXR-α, ABCA-1 and PPAR-γ but not of PPAR-α (Figure 2). OxLDL in the plaque correlated inversely with the expression of SOD1 (R2=0.55; P=0.0006), LXR-α (R2=0.44; P=0.004), PPAR-γ (R2=0.37; P=0.01), ABCA-1 (R2=0.33; P=0.02) and CD36 (R2=0.29; P=0.03).
Figure 2.
Expression of SOD1 (a), CD36 (b), LXR-α (c), ABCA-1 (d), PPAR-α (e) and PPAR-γ (f) in the aorta of DKO mice at 12 weeks (n=8) and in rosuvastatin treated (n=6) and control (n=11) DKO mice at 24 weeks. *P<0.05 and **P<0.01.
Figure 3a shows the colocalization of macrophages, ox-LDL and SOD1 protein. The rosuvastatin-associated increase in SOD1 mRNA expression in the aorta was associated with a 2.7-fold increase in SOD1 protein in the plaques (Figure 3b). Western blotting of cell lysates from aortas showed that SOD1 protein was lower in control DKO mice (72%) compared with C57BL6 (100%), and that rosuvastatin increased SOD1 (117%) (Figure 3c).
SOD1 expression in THP-1 cells
We investigated the effect of oxLDL-induced foam cell formation on SOD1 expression in THP-1 cells because SOD1 was the strongest inverse correlate of oxLDL in the aortas. Figure 4b shows that SOD1 expression was 21% lower in THP-1 macrophages and 30% lower in THP-1 foam cells. In the presence of 0.5 μM rosuvastatin, SOD1 expression in macrophages was 15% higher and that in foam cells was 13% higher. At 2.5 μM, rosuvastatin restored the expression of SOD1 in THP-1 macrophages and foam cells.
Discussion
The aim of the present study was to elucidate the mechanism of the inhibitory effect of rosuvastatin on the accumulation of plaque ox-LDL and on plaque volume in the absence of cholesterol lowering in a strain of mice (DKO) that exhibits most of the components of the metabolic syndrome. Rosuvastatin lowered plasma triglycerides and FFA. This was associated with an increase in insulin sensitivity independent of cholesterol lowering. Rosuvastatin lowered plaque volume, via a reduction in plaque macrophages, lipids and ox-LDL, and increased the SMC-to-macrophage ratio. Rosuvastatin increased the SOD1 RNA and protein in agreement with a reduction of plaque ox-LDL. It also increased CD36, LXR-α and ABCA-1 expression, in agreement with a lower ox-LDL and lipid content. The increased expression of SOD1, CD36 and LXR-α and ABCA-1 was observed in the aorta despite a lower number of macrophages and in the absence of an effect on total cholesterol and lipoprotein distribution. Furthermore, oxLDL-induced foam cell formation lowered SOD1 expression in THP-1 macrophages. Rosuvastatin restored this expression.
Effect of rosuvastatin on atherosclerosis
It is well known that statins efficiently lower the progression rate of atherosclerosis and stabilize plaques in man (Crisby et al., 2001; Libby and Aikawa, 2003; Rosenson, 2004). In agreement with this concept, rosuvastatin not only decreased plaque volume, but changed plaque composition.
We, among others, have demonstrated an association between the oxidation of LDL and coronary artery disease (Holvoet et al., 1998; Tsimikas et al., 2005). We have shown that obesity, diabetes and dyslipidemia are associated with high levels of oxLDL before clinical evidence of cardiovascular disease in patients with the metabolic syndrome (Holvoet et al., 2004). Because the assay for oxLDL in blood is based on a mouse monoclonal antibody, oxLDL cannot be measured directly in mouse blood. Previously, the titer of autoantibody against MDA-modified LDL has been used as a proxy for oxLDL in mice (Tsimikas et al., 2001; Verreth et al., 2004; Holvoet et al., 2006; Mackness et al., 2006). Compared with ob/ob and LDLR−/− mice, the DKO mice had higher titers of autoantibody. Rosuvastatin did not reduce the antibody titer, suggesting that it can reduce oxLDL in the plaque without measurable effects on oxLDL in the circulation. Rosuvastatin not only lowered the oxLDL area in the plaque, it also reduced the oxLDL-to-LDL ratio in the plaque. This suggests that rosuvastatin reduced the oxidation of LDL and/or increased the removal of oxLDL from the plaques. Rosuvastatin also decreased lipids suggesting that it also increased lipid efflux. We then searched for genes that could be involved in reducing the plaque oxLDL and lipids.
Effect of rosuvastatin on gene expression in the aorta
We show here that SOD1 expression in aortas from DKO mice is lower than in that from lean mice from the age of 12 weeks onwards. The lower SOD1 expression was associated with increased deposition of oxLDL in the plaques from 24-week old DKO mice. SOD1 RNA and protein expressions in the aortas of rosuvastatin-treated mice were higher than in control DKO mice. The rosuvastatin-induced restoration of SOD1 expression was associated with a decrease of plaque oxLDL. It is however unclear if a reduction in SOD1 is then associated with an increase in oxLDL, or if an increase in oxLDL results in a reduction in SOD1. In preliminary experiments (data not shown) we found that SOD1 expression in the aortas of 6-week-old DKO mice is lower than in C57BL6 control mice. This is before oxLDL is present in the circulation or in the plaque, suggesting that a reduction in SOD1 could be responsible for an increase in oxLDL.
Previously, it has been demonstrated that atorvastatin treatment activated PPAR-γ and increased LXR-mediated gene expression suggesting that atorvastatin induces cholesterol efflux through a molecular cascade involving inhibition of RhoA signaling, leading to increased PPAR-γ activity, enhanced LXR activation, increased ABCA-1 expression and cholesterol efflux. Finally, statin treatment inhibited cholesteryl ester accumulation in macrophages challenged with atherogenic hypertriglyceridemic very LDLs indicating that statins can regulate foam cell formation (Argmann et al., 2005). Thus the observed increased expression of PPAR-γ, LXR, ABCA-1 and the lower amount of plaque lipids in rosuvastatin-treated DKO mice is in agreement with that mechanism. Statins also increased the activity of PPAR-γ, but not PPAR-α, in the aortas of hypercholesterolemic rabbits (Umeji et al., 2006). Finally, the concomitant increase in CD36 and ABCA-1 is in agreement with the previous finding that removal of oxLDL from the vascular wall is mediated by macrophages by increasing the uptake of oxLDL via CD36 and removing cholesterol via ABCA-1 (Chawla et al., 2001).
The increase in PPAR-γ expression in rosuvastatin-treated mice could also be the result of the lower lipid levels and the higher insulin sensitivity. Weight loss that was associated with a similar lowering in triglycerides and FFA, and a similar improvement in insulin sensitivity in the absence of cholesterol lowering, also induced PPAR-γ expression in the aorta (Verreth et al., 2004). However, treatment with a dual PPAR-α/γ agonist that lowered FFA and improved insulin sensitivity, but had no effect on triglycerides and cholesterol, also had no effect on PPAR-γ expression, plaque volume and oxLDL (Verreth et al., 2006). Overall our data suggest that overexpression of PPAR-γ in the vascular wall, even independently of its effect on FFA and insulin sensitivity is a crucial mechanism for preventing the accumulation of ox-LDL and plaque progression. However, we cannot exclude the possibility that the decrease in triglycerides after rosuvastatin-treatment and weight loss is partially responsible for the increase in PPAR-γ in the vascular wall in these mice.
Effect of rosuvastatin on gene expression in THP-1 cells
As mentioned, SOD1 expression in the aorta of 6-week-old DKO mice was lower compared to that in lean C57BL6 mice. This is before oxLDL is present in the circulation or in the plaque, indicating that a reduction in SOD1 could be responsible for an increase in oxLDL. However, our data do not exclude that oxLDL by itself decreases the SOD1 expression. Therefore, we investigated the effect of THP-1 macrophage differentiation and oxLDL-induced foam cell generation on SOD1 expression. We now show that in macrophages SOD1 expression was decreased compared to THP-1 monocytes, and that oxLDL-induced foam cell formation lowered SOD1 expression even more. Interestingly, rosuvastatin prevented this decrease in a dosage-dependent manner. The exact role of SOD1 in macrophages in regulating oxidative stress and its interaction with oxLDL in circulation and in plaques warrants further investigation. Therefore, we are now performing bone marrow transplantation from mice deficient in SOD1 and from SOD1 transgenic mice in DKO mice.
Relevance of this study
The inverse relationship between SOD1 expression and ox-LDL in plaque is a relevant finding because oxidative stress contributes to post-ischaemic injury in the heart and because increasing SOD1 protects against this increased oxidative stress (Wang et al., 1998; Tanaka et al., 2004; Fukushima et al., 2006). The observed induction of PPAR-γ in the aorta and the inverse relation with oxLDL in the plaque adds to its important role in regulating oxidative stress as well as inflammation. In vitro studies, animal models and clinical studies indicate that PPAR-γ agonists suppress chronic inflammatory processes, thereby improving endothelial function, and that they enhance plaque stabilization and regression (Staels, 2005). Here, we propose that the induction of SOD1, possibly through induction of PPAR-γ, is an important mechanism for preventing oxidation of LDL in the arterial wall.
Limitations of the study
Total RNA extracts and total cell lysates of the aorta were used to determine the mRNA expression and protein expression, respectively, in the aorta. Therefore we could not determine which cells contributed to the observed changes in expression. However, immunohistochemical analysis in placebo and rosuvastatin-treated DKO mice showed co-localization of macrophages, oxLDL and SOD1. Our recent finding that SOD1 was the strongest inverse correlate of oxLDL in plaque macrophages isolated from coronary plaques of hypercholesterolaemic pigs by laser capture microdissection further supports the interaction between oxLDL and SOD1 in the atherosclerotic plaque (Holvoet et al., 2006).
Conclusions
Rosuvastatin inhibited atherosclerosis and changed plaque composition in the aortic arch of obese, dyslipidemic mice at a dosage that did not affect cholesterol but increased insulin sensitivity. It decreased oxLDL in the aortic arch without affecting circulating oxLDL. We have identified SOD1 as a potentially important mediator of the prevention of oxLDL accumulation within atherosclerotic plaques. Our data also support the hypothesis that oxLDL lowers the anti-oxidative defence of macrophages by lowering SOD1 expression, and that rosuvastatin is able to restore the antioxidant defence by restoring SOD1 expression.
Acknowledgments
This study was supported in part by the Fonds voor Wetenschappelijk Onderzoek–Vlaanderen (Program G027604), the Interuniversity Attraction Poles Program – Belgian Science Policy (P5/02). Dieuwke De Keyzer is a researcher funded by a PhD grant of the Institute for the Promotion of Innovation Through Science and Technology in Flanders (IWT-Vlaanderen). Wim Verreth is supported by a post-doctoral grant and Benjamine Geeraert by a pre-doctoral grant of the Bijzonder Onderzoeksfonds of KU Leuven. We thank Hilde Bernar, Els Deridder, and Michèle Landeloos for excellent technical assistance.
Abbreviations
- ABCA-1
ATP-binding cassette transporter A1
- AUC
area under the curve
- CD36
fatty acid translocase
- Ct
threshold cycle
- DKO mice
double knockout mice with combined leptin and LDL-receptor deficiency
- FFA
free-fatty acids
- HOMA
homeostasis model assessment
- LDL
low-density lipoproteins
- LXR-α
liver X receptor α
- MDA
malondialdehyde
- oxLDL
oxidized LDL
- PPAR
peroxisome proliferator–activated receptor
- ROS
reactive-oxygen species
- SMC
smooth muscle cell
- SOD
superoxide dismutase
- SOD1
copper–zinc SOD
- SOD2
manganese SOD
- SOD3
extracellular SOD
Conflict of interest
We received an unrestricted grant from AstraZeneca UK. GS is an employee of AstraZeneca UK.
References
- Argmann CA, Edwards JY, Sawyez CG, O'Neil CH, Hegele RA, Pickering JG, et al. Regulation of macrophage cholesterol efflux through hydroxymethylglutaryl-CoA reductase inhibition: a role for RhoA in ABCA1-mediated cholesterol efflux. J Biol Chem. 2005;280:22212–22221. doi: 10.1074/jbc.M502761200. [DOI] [PubMed] [Google Scholar]
- Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- Costa A, Casamitjana R, Casals E, Alvarez L, Morales J, Masramon X, et al. Effects of atorvastatin on glucose homeostasis, postprandial triglyceride response and C-reactive protein in subjects with impaired fasting glucose. Diabet Med. 2003;20:743–745. doi: 10.1046/j.1464-5491.2003.00993.x. [DOI] [PubMed] [Google Scholar]
- Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 2001;103:926–933. doi: 10.1161/01.cir.103.7.926. [DOI] [PubMed] [Google Scholar]
- Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- Fang X, Weintraub NL, Rios CD, Chappell DA, Zwacka RM, Engelhardt JF, et al. Overexpression of human superoxide dismutase inhibits oxidation of low-density lipoprotein by endothelial cells. Circ Res. 1998;82:1289–1297. doi: 10.1161/01.res.82.12.1289. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- Fukushima S, Coppen SR, Varela-Carver A, Brindley G, Yamahara K, Sarathchandra P, et al. Enhanced efficiency of superoxide dismutase-induced cardioprotection by retrograde intracoronary administration. Cardiovasc Res. 2006;69:459–465. doi: 10.1016/j.cardiores.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Grip O, Janciauskiene S, Lindgren S. Atorvastatin activates PPAR-gamma and attenuates the inflammatory response in human monocytes. Inflamm Res. 2002;51:58–62. doi: 10.1007/BF02684000. [DOI] [PubMed] [Google Scholar]
- Guclu F, Ozmen B, Hekimsoy Z, Kirmaz C. Effects of a statin group drug, pravastatin, on the insulin resistance in patients with metabolic syndrome. Biomed Pharmacother. 2004;58:614–618. doi: 10.1016/j.biopha.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Hasty AH, Shimano H, Osuga J, Namatame I, Takahashi A, Yahagi N, et al. Severe hypercholesterolemia, hypertriglyceridemia, and atherosclerosis in mice lacking both leptin and the low density lipoprotein receptor. J Biol Chem. 2001;276:37402–37408. doi: 10.1074/jbc.M010176200. [DOI] [PubMed] [Google Scholar]
- Holvoet P, Davey PC, De KD, Doukoure M, Deridder E, Bochaton-Piallat ML, et al. Oxidized low-density lipoprotein correlates positively with toll-like receptor 2 and interferon regulatory factor-1 and inversely with superoxide dismutase-1 expression: studies in hypercholesterolemic swine and THP-1 cells. Arterioscler Thromb Vasc Biol. 2006;26:1558–1565. doi: 10.1161/01.ATV.0000226553.01555.02. [DOI] [PubMed] [Google Scholar]
- Holvoet P, Kritchevsky SB, Tracy RP, Mertens A, Rubin SM, Butler J, et al. The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes. 2004;53:1068–1073. doi: 10.2337/diabetes.53.4.1068. [DOI] [PubMed] [Google Scholar]
- Holvoet P, Vanhaecke J, Janssens S, Van de WF, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- Inoue I, Goto S, Mizotani K, Awata T, Mastunaga T, Kawai S, et al. Lipophilic HMG-CoA reductase inhibitor has an anti-inflammatory effect: reduction of MRNA levels for interleukin-1beta, interleukin-6, cyclooxygenase-2, and p22phox by regulation of peroxisome proliferator-activated receptor alpha (PPARalpha) in primary endothelial cells. Life Sci. 2000;67:863–876. doi: 10.1016/s0024-3205(00)00680-9. [DOI] [PubMed] [Google Scholar]
- Kleemann R, Princen HM, Emeis JJ, Jukema JW, Fontijn RD, Horrevoets AJ, et al. Rosuvastatin reduces atherosclerosis development beyond and independent of its plasma cholesterol-lowering effect in APOE*3-Leiden transgenic mice: evidence for antiinflammatory effects of rosuvastatin. Circulation. 2003;108:1368–1374. doi: 10.1161/01.CIR.0000086460.55494.AF. [DOI] [PubMed] [Google Scholar]
- Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. Am J Cardiol. 2003;91:4B–8B. doi: 10.1016/s0002-9149(02)03267-8. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbye JB, Thompson PD. Statin use in the metabolic syndrome. Curr Atheroscler Rep. 2005;7:17–21. doi: 10.1007/s11883-005-0070-9. [DOI] [PubMed] [Google Scholar]
- Mackness B, Quarck R, Verreth W, Mackness M, Holvoet P. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1545–1550. doi: 10.1161/01.ATV.0000222924.62641.aa. [DOI] [PubMed] [Google Scholar]
- Mertens A, Verhamme P, Bielicki JK, Phillips MC, Quarck R, Verreth W, et al. Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice: LCAT gene transfer decreases atherosclerosis. Circulation. 2003;107:1640–1646. doi: 10.1161/01.CIR.0000056523.08033.9F. [DOI] [PubMed] [Google Scholar]
- Paniagua JA, Lopez-Miranda J, Escribano A, Berral FJ, Marin C, Bravo D, et al. Cerivastatin improves insulin sensitivity and insulin secretion in early-state obese type 2 diabetes. Diabetes. 2002;51:2596–2603. doi: 10.2337/diabetes.51.8.2596. [DOI] [PubMed] [Google Scholar]
- Parker RA, Huang Q, Tesfamariam B. Influence of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors on endothelial nitric oxide synthase and the formation of oxidants in the vasculature. Atherosclerosis. 2003;169:19–29. doi: 10.1016/s0021-9150(03)00100-x. [DOI] [PubMed] [Google Scholar]
- Rosenson RS. Statins in atherosclerosis: lipid-lowering agents with antioxidant capabilities. Atherosclerosis. 2004;173:1–12. doi: 10.1016/S0021-9150(03)00239-9. [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents. Circulation. 2004;109:II18–II26. doi: 10.1161/01.CIR.0000129505.34151.23. [DOI] [PubMed] [Google Scholar]
- Sonmez A, Baykal Y, Kilic M, Yilmaz MI, Saglam K, Bulucu F, et al. Fluvastatin improves insulin resistance in nondiabetic dyslipidemic patients. Endocrine. 2003;22:151–154. doi: 10.1385/endo:22:2:151. [DOI] [PubMed] [Google Scholar]
- Staels B. PPARgamma and atherosclerosis. Curr Med Res Opin. 2005;21 Suppl 1:S13–S20. doi: 10.1185/030079905X36440. [DOI] [PubMed] [Google Scholar]
- Stoll LL, McCormick ML, Denning GM, Weintraub NL. Antioxidant effects of statins. Timely Top Med Cardiovasc Dis. 2005;9:E1. [PubMed] [Google Scholar]
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Mokhtari GK, Terry RD, Balsam LB, Lee KH, Kofidis T, et al. Overexpression of human copper/zinc superoxide dismutase (SOD1) suppresses ischemia-reperfusion injury and subsequent development of graft coronary artery disease in murine cardiac grafts. Circulation. 2004;110:II200–II206. doi: 10.1161/01.CIR.0000138390.81640.54. [DOI] [PubMed] [Google Scholar]
- Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- Tsimikas S, Palinski W, Witztum JL. Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:95–100. doi: 10.1161/01.atv.21.1.95. [DOI] [PubMed] [Google Scholar]
- Umeji K, Umemoto S, Itoh S, Tanaka M, Kawahara S, Fukai T, et al. Comparative effects of pitavastatin and probucol on oxidative stress, Cu/Zn superoxide dismutase, PPAR-gamma, and aortic stiffness in hypercholesterolemia. Am J Physiol Heart Circ Physiol. 2006;291:H2522–H2532. doi: 10.1152/ajpheart.01198.2005. [DOI] [PubMed] [Google Scholar]
- Usui S, Hara Y, Hosaki S, Okazaki M. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J Lipid Res. 2002;43:805–814. [PubMed] [Google Scholar]
- van Eck M, Twisk J, Hoekstra M, Van Rij BT, Van der Lans CA, Bos IS, et al. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J Biol Chem. 2003;278:23699–23705. doi: 10.1074/jbc.M211233200. [DOI] [PubMed] [Google Scholar]
- Verreth W, De KD, Pelat M, Verhamme P, Ganame J, Bielicki JK, et al. Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation. 2004;110:3259–3269. doi: 10.1161/01.CIR.0000147614.85888.7A. [DOI] [PubMed] [Google Scholar]
- Verreth W, Ganame J, Mertens A, Bernar H, Herregods MC, Holvoet P. Peroxisome Proliferator-Activated Receptor-{alpha},{gamma}-Agonist Improves Insulin Sensitivity and Prevents Loss of Left Ventricular Function in Obese, Dyslipidemic Mice. Arterioscler Thromb Vasc Biol. 2006;26:922–928. doi: 10.1161/01.ATV.0000207318.42066.bb. [DOI] [PubMed] [Google Scholar]
- Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol. 2000;20:61–69. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- Wang P, Chen H, Qin H, Sankarapandi S, Becher MW, Wong PC, et al. Overexpression of human copper, zinc-superoxide dismutase (SOD1) prevents postischemic injury. Proc Natl Acad Sci USA. 1998;95:4556–4560. doi: 10.1073/pnas.95.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]