Abstract
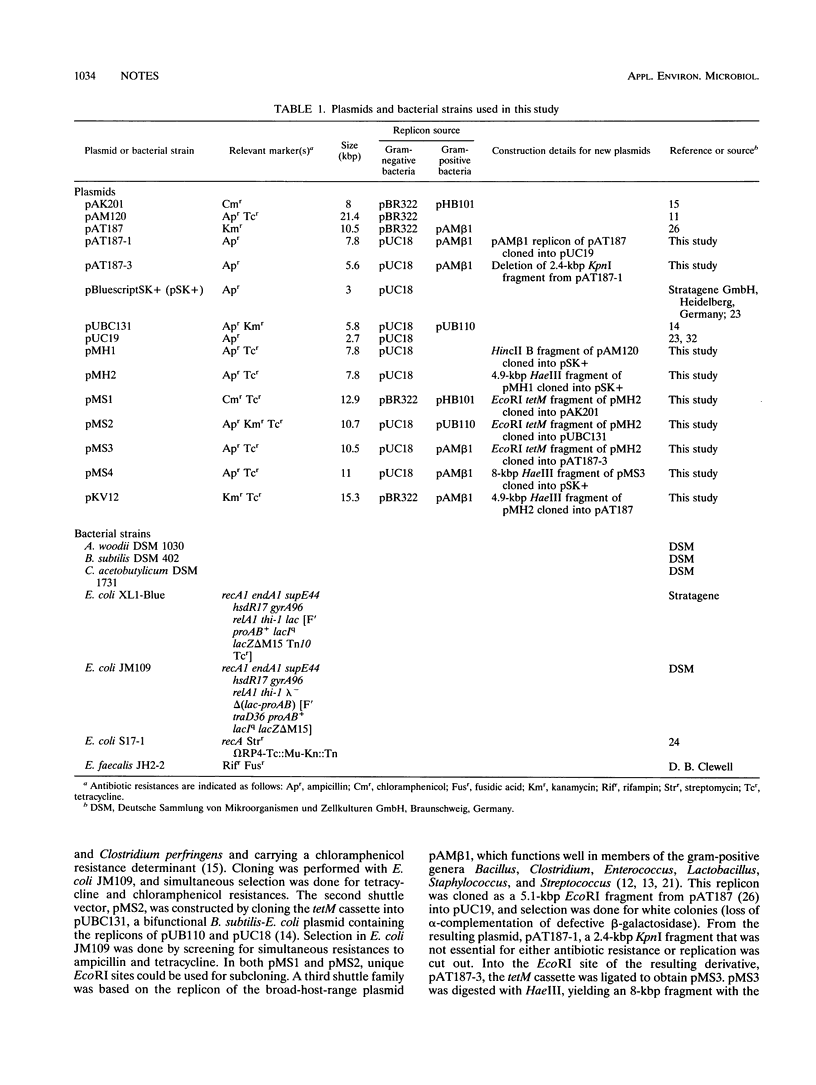
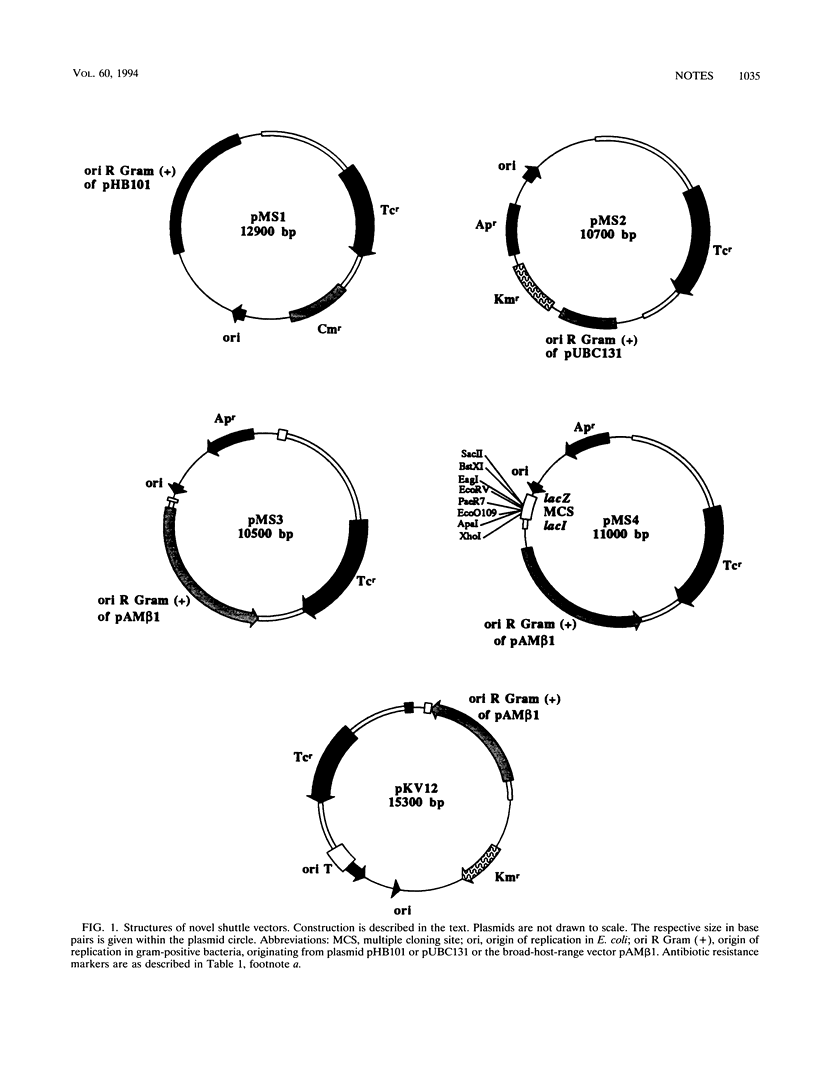
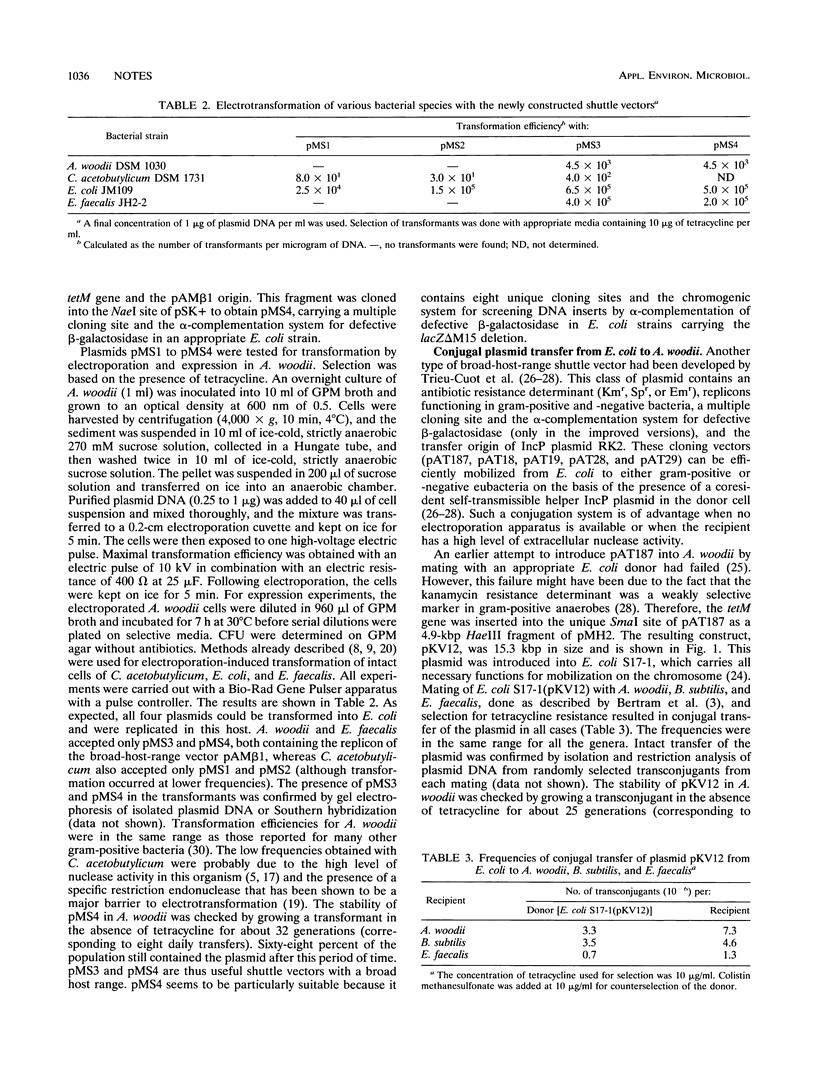
Shuttle vectors (pMS3 and pMS4) which replicated in Escherichia coli and in gram-positive Acetobacterium woodii were constructed by ligating the replication origin of plasmid pAMβ1 with the E. coli cloning vector pUC19 and the tetM gene of streptococcal transposon Tn916. Electrotransformation of A. woodii was achieved at frequencies of 4.5 × 103 transformants per μg of plasmid DNA. For conjugal plasmid transfer, the mobilizable shuttle vector pKV12 was constructed by cloning the tetM gene into pAT187. Mating of E. coli containing pKV12 with A. woodii resulted in transfer frequencies of 3 × 10-6 to 7 × 10-6 per donor or recipient.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram J., Strätz M., Dürre P. Natural transfer of conjugative transposon Tn916 between gram-positive and gram-negative bacteria. J Bacteriol. 1991 Jan;173(2):443–448. doi: 10.1128/jb.173.2.443-448.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschhorn H., Dürre P., Gottschalk G. Production and Utilization of Ethanol by the Homoacetogen Acetobacterium woodii. Appl Environ Microbiol. 1989 Jul;55(7):1835–1840. doi: 10.1128/aem.55.7.1835-1840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Rodz A. L., Gilmore M. S. High efficiency introduction of plasmid DNA into glycine treated Enterococcus faecalis by electroporation. Mol Gen Genet. 1990 Oct;224(1):152–154. doi: 10.1007/BF00259462. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horaud T., Le Bouguenec C., Pepper K. Molecular genetics of resistance to macrolides, lincosamides and streptogramin B (MLS) in streptococci. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):111–135. doi: 10.1093/jac/16.suppl_a.111. [DOI] [PubMed] [Google Scholar]
- Jannière L., Bruand C., Ehrlich S. D. Structurally stable Bacillus subtilis cloning vectors. Gene. 1990 Mar 1;87(1):53–61. doi: 10.1016/0378-1119(90)90495-d. [DOI] [PubMed] [Google Scholar]
- Kim A. Y., Blaschek H. P. Construction of an Escherichia coli-Clostridium perfringens shuttle vector and plasmid transformation of Clostridium perfringens. Appl Environ Microbiol. 1989 Feb;55(2):360–365. doi: 10.1128/aem.55.2.360-365.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Blaschek H. P. Transformation of Heat-Treated Clostridium acetobutylicum Protoplasts with pUB110 Plasmid DNA. Appl Environ Microbiol. 1984 Oct;48(4):737–742. doi: 10.1128/aem.48.4.737-742.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Mermelstein L. D., Welker N. E., Bennett G. N., Papoutsakis E. T. Expression of cloned homologous fermentative genes in Clostridium acetobutylicum ATCC 824. Biotechnology (N Y) 1992 Feb;10(2):190–195. doi: 10.1038/nbt0292-190. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W. Enzymology of the acetyl-CoA pathway of CO2 fixation. Crit Rev Biochem Mol Biol. 1991;26(3-4):261–300. doi: 10.3109/10409239109114070. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Carlier C., Poyart-Salmeron C., Courvalin P. A pair of mobilizable shuttle vectors conferring resistance to spectinomycin for molecular cloning in Escherichia coli and in gram-positive bacteria. Nucleic Acids Res. 1990 Jul 25;18(14):4296–4296. doi: 10.1093/nar/18.14.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Carlier C., Poyart-Salmeron C., Courvalin P. Shuttle vectors containing a multiple cloning site and a lacZ alpha gene for conjugal transfer of DNA from Escherichia coli to gram-positive bacteria. Gene. 1991 Jun 15;102(1):99–104. doi: 10.1016/0378-1119(91)90546-n. [DOI] [PubMed] [Google Scholar]
- Wood H. G. Life with CO or CO2 and H2 as a source of carbon and energy. FASEB J. 1991 Feb;5(2):156–163. doi: 10.1096/fasebj.5.2.1900793. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


