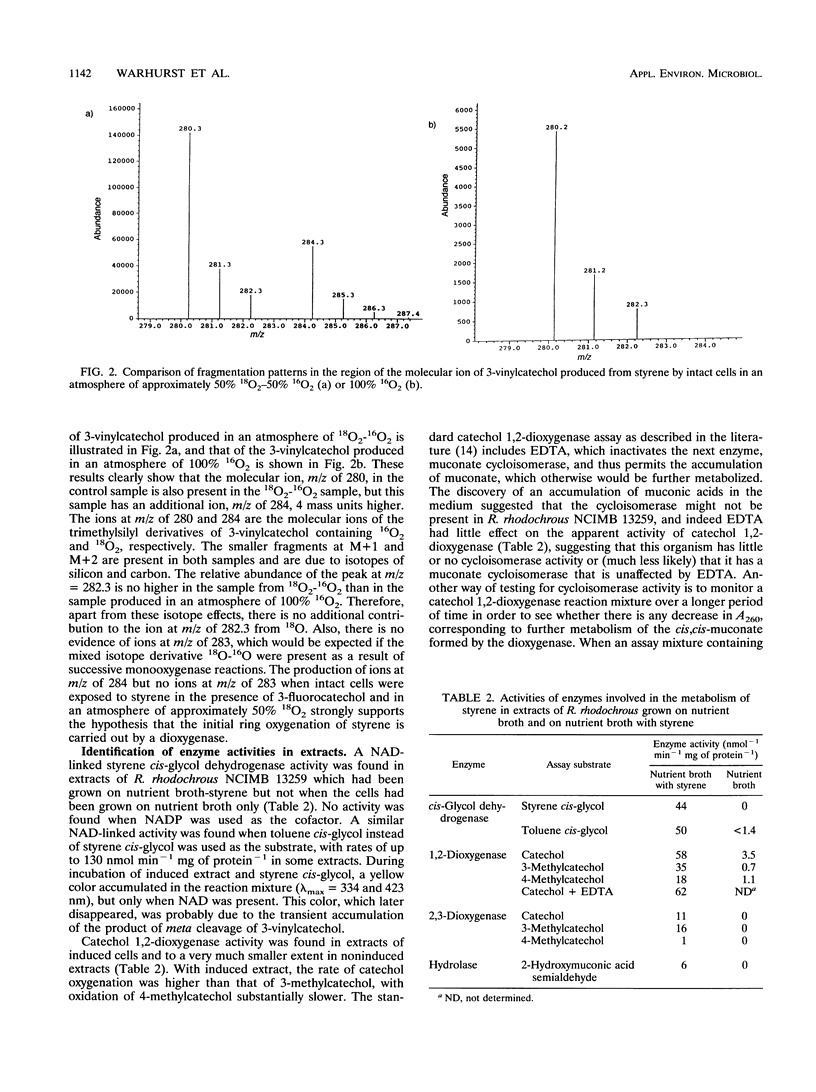
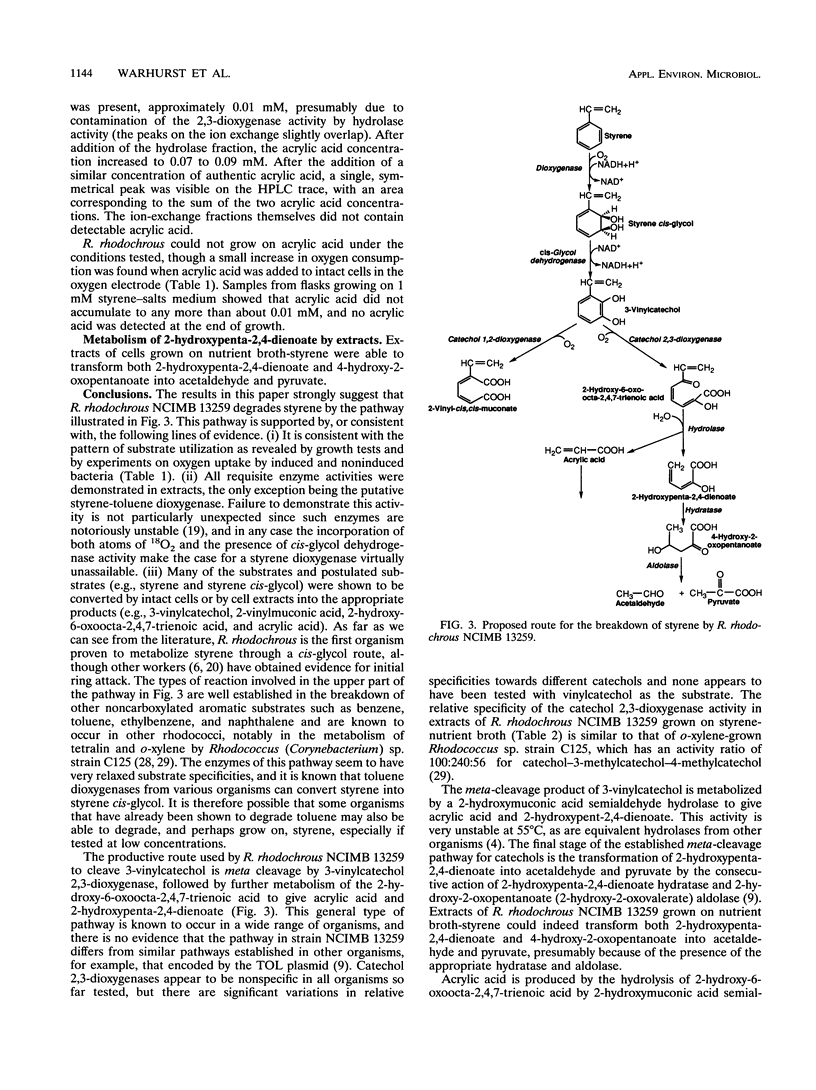
Abstract
Rhodococcus rhodochrous NCIMB 13259 grows on styrene, toluene, ethylbenzene, and benzene as sole carbon sources. Simultaneous induction tests with cells grown on styrene or toluene showed high rates of oxygen consumption with toluene cis-glycol and 3-methylcatechol, suggesting the involvement of a cis-glycol pathway. 3-Vinylcatechol accumulated when intact cells were incubated with styrene in the presence of 3-fluorocatechol to inhibit catechol dioxygenase activity. Experiments with 18O2 showed that 3-vinylcatechol was produced following a dioxygenase ring attack. Extracts contained a NAD-dependent cis-glycol dehydrogenase, which converted styrene cis-glycol to 3-vinylcatechol. Both catechol 1,2- and 2,3-dioxygenase activities were present, and these were separated from each other and from the activities of cis-glycol dehydrogenase and 2-hydroxymuconic acid semialdehyde hydrolase by ion-exchange chromatography of extracts. 2-Vinylmuconate accumulated in the growth medium when cells were grown on styrene, apparently as a dead-end product, and extracts contained no detectable muconate cycloisomerase activity. 3-Vinylcatechol was cleaved by catechol 2,3-dioxygenase to give a yellow compound, tentatively identified as 2-hydroxy-6-oxoocta-2,4,7-trienoic acid, and the action of 2-hydroxymuconic acid semialdehyde hydrolase on this produced acrylic acid. A compound with the spectral characteristics of 2-hydroxypenta-2,4-dienoate was produced by the action of 2-hydroxymuconic acid semialdehyde hydrolase on the 2,3-cleavage product of 3-methylcatechol. Extracts were able to transform 2-hydroxypenta-2,4-dienoate and 4-hydroxy-2-oxopentanoate into acetaldehyde and pyruvate.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels I., Knackmuss H. J., Reineke W. Suicide Inactivation of Catechol 2,3-Dioxygenase from Pseudomonas putida mt-2 by 3-Halocatechols. Appl Environ Microbiol. 1984 Mar;47(3):500–505. doi: 10.1128/aem.47.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs J. D., Fewson C. A. Regulation of synthesis of benzyl alcohol dehydrogenase in Acinetobacter calcoaceticus NCIB8250. J Gen Microbiol. 1977 Nov;103(1):127–140. doi: 10.1099/00221287-103-1-127. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burlage R. S., Hooper S. W., Sayler G. S. The TOL (pWW0) catabolic plasmid. Appl Environ Microbiol. 1989 Jun;55(6):1323–1328. doi: 10.1128/aem.55.6.1323-1328.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinsworth W. L., Chapman P. J., Dagley S. Stereospecific enzymes in the degradation of aromatic compounds by pseudomonas putida. J Bacteriol. 1973 Feb;113(2):922–931. doi: 10.1128/jb.113.2.922-931.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps R. E., Trudgill P. W., Whateley J. G. The metabolism of 1-phenylethanol and acetophenone by Nocardia T5 and an Arthrobacter species. Eur J Biochem. 1978 May;86(1):175–186. doi: 10.1111/j.1432-1033.1978.tb12297.x. [DOI] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grbić-Galić D., Churchman-Eisel N., Mraković I. Microbial transformation of styrene by anaerobic consortia. J Appl Bacteriol. 1990 Aug;69(2):247–260. doi: 10.1111/j.1365-2672.1990.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Haigler B. E., Gibson D. T. Purification and properties of NADH-ferredoxinNAP reductase, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990 Jan;172(1):457–464. doi: 10.1128/jb.172.1.457-464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmans S., Smits J. P., van der Werf M. J., Volkering F., de Bont J. A. Metabolism of Styrene Oxide and 2-Phenylethanol in the Styrene-Degrading Xanthobacter Strain 124X. Appl Environ Microbiol. 1989 Nov;55(11):2850–2855. doi: 10.1128/aem.55.11.2850-2855.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmans S., van der Werf M. J., de Bont J. A. Bacterial degradation of styrene involving a novel flavin adenine dinucleotide-dependent styrene monooxygenase. Appl Environ Microbiol. 1990 May;56(5):1347–1351. doi: 10.1128/aem.56.5.1347-1351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn M., Wodarz R., Schoknecht W., Weichardt H., Bayer E. Styrene metabolism in man: gas chromatographic separation of mandelic acid enantiomers in the urine of exposed persons. Arch Toxicol. 1984 Mar;55(1):59–63. doi: 10.1007/BF00316587. [DOI] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- Schraa G., Bethe B. M., van Neerven A. R., Van den Tweel W. J., Van der Wende E., Zehnder A. J. Degradation 1,2-dimethylbenzene by Corynebacterium strain C125. Antonie Van Leeuwenhoek. 1987;53(3):159–170. doi: 10.1007/BF00393844. [DOI] [PubMed] [Google Scholar]
- Sikkema J., de Bont J. A. Metabolism of tetralin (1,2,3,4-tetrahydronaphthalene) in Corynebacterium sp. strain C125. Appl Environ Microbiol. 1993 Feb;59(2):567–572. doi: 10.1128/aem.59.2.567-572.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis M. G., Chapman S. K. Isolation and partial characterization of an extradiol non-haem iron dioxygenase which preferentially cleaves 3-methylcatechol. Biochem J. 1990 Mar 1;266(2):605–609. [PMC free article] [PubMed] [Google Scholar]
- Yeh W. K., Gibson D. T., Liu T. N. Toluene dioxygenase: a multicomponent enzyme system. Biochem Biophys Res Commun. 1977 Sep 9;78(1):401–410. doi: 10.1016/0006-291x(77)91268-2. [DOI] [PubMed] [Google Scholar]


