Abstract
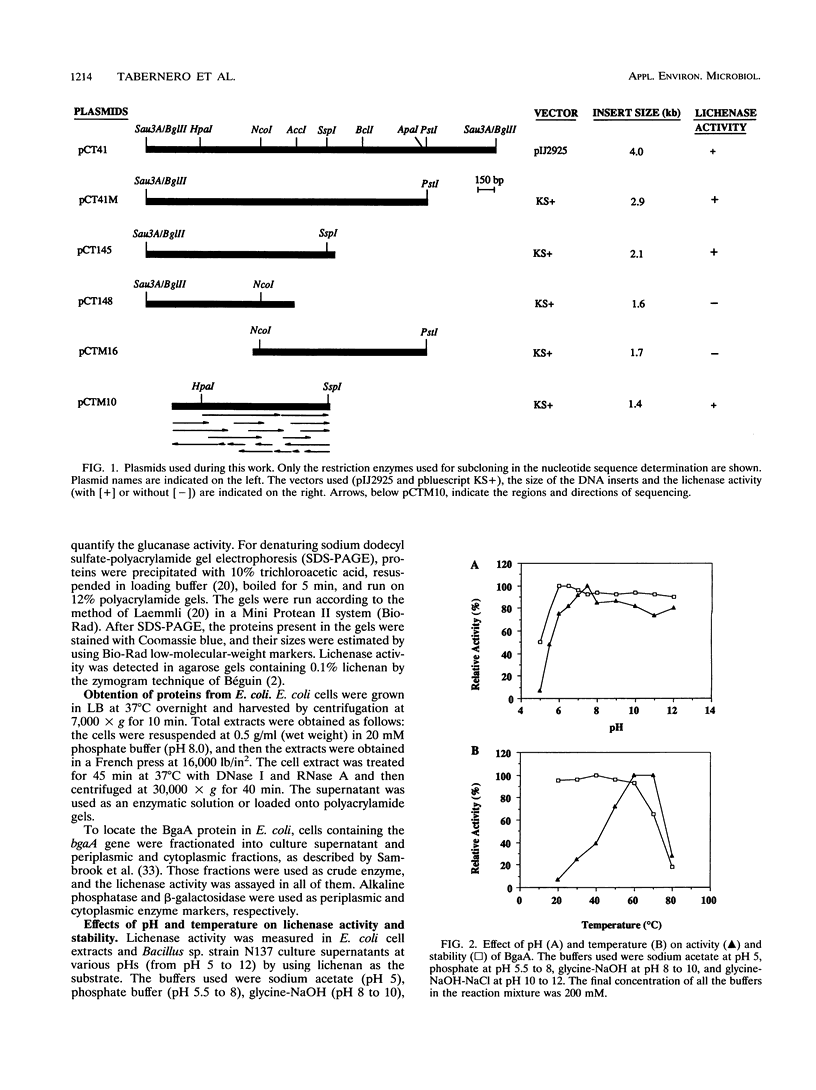
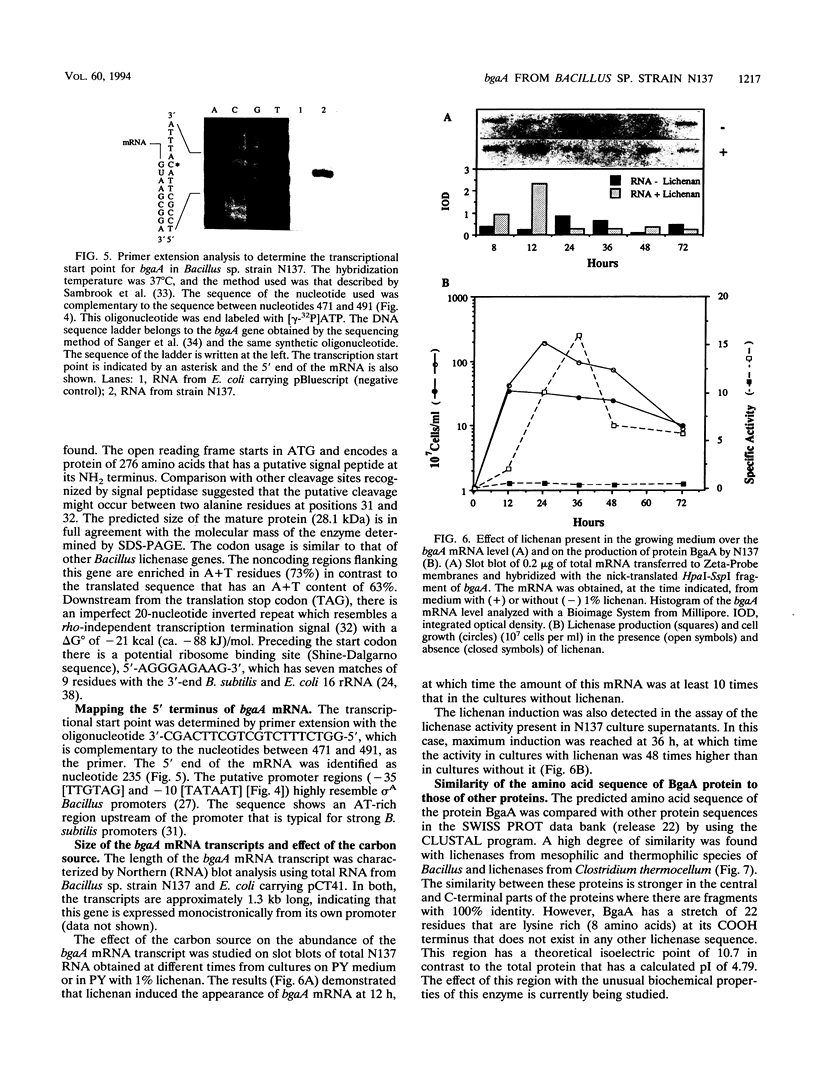
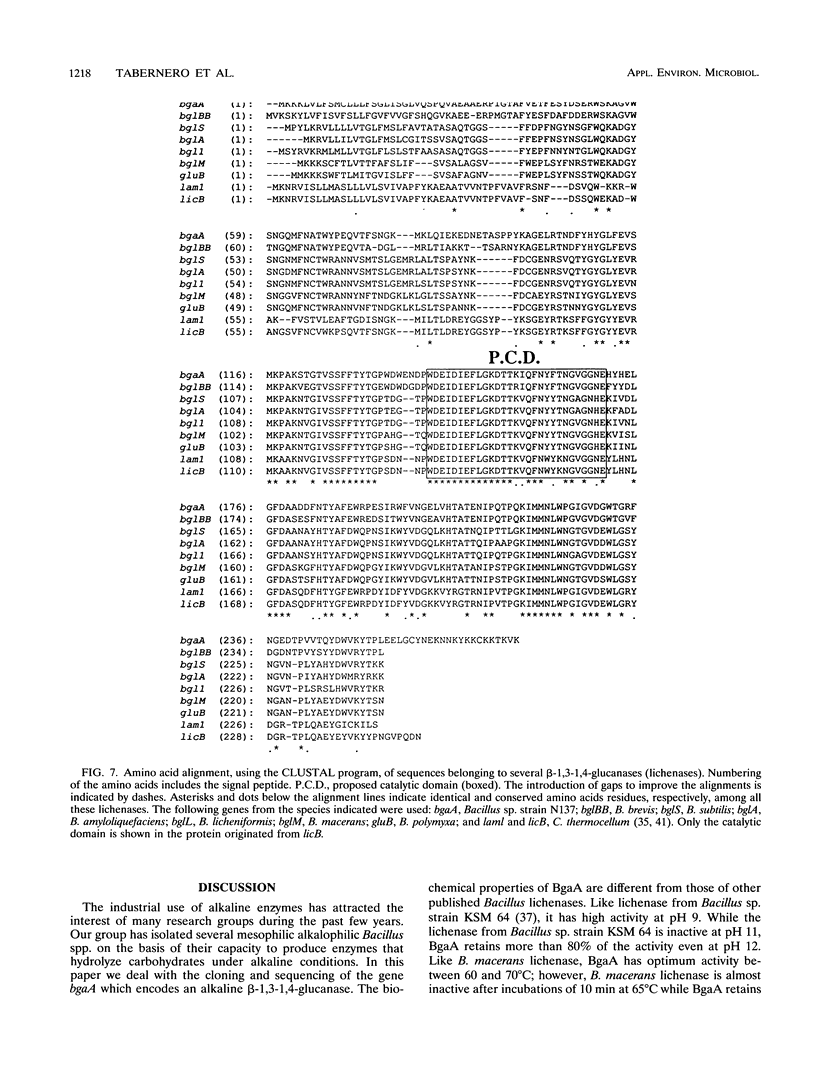
The gene bgaA encoding an alkaline endo-beta-1,3-1,4-glucanase (lichenase) from an alkalophilic Bacillus sp. strain N137, isolated in our laboratory, was cloned and expressed from its own promoter in Escherichia coli. The nucleotide sequence of a 1,416-bp DNA fragment containing bgaA was determined and revealed an open reading frame of 828 nucleotides. The deduced protein sequence consists of 276 amino acids and has a 31-amino-acid putative signal peptide which is functional in E. coli, in which the BgaA protein is located mainly in the periplasmic space. The lichenase activity of BgaA is stable between pH 6 and 12, it shows optimal activity at a temperature between 60 and 70 degrees C, and it retains 65% of its activity after incubation at 70 degrees C for 1 h. This protein is similar to some other lichenases from Bacillus species such as B. amyloliquefaciens, B. brevis, B. licheniformis, B. macerans, B. polymyxa, and B. subtilis. However, it has a lysine-rich region at the carboxy terminus which is not found in any other published lichenase sequence and might be implicated in the unusual biochemical properties of this enzyme. The location of the mRNA 5' end was determined by primer extension and corresponds to nucleotide 235. A typical Bacillus sigma A promoter precedes the transcription start site.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriss R., Buettner K., Maentsaelae P. Structure of the beta-1,3-1,4-glucanase gene of Bacillus macerans: homologies to other beta-glucanases. Mol Gen Genet. 1990 Jul;222(2-3):278–283. doi: 10.1007/BF00633829. [DOI] [PubMed] [Google Scholar]
- Bueno A., Vazquez de Aldana C. R., Correa J., Villa T. G., del Rey F. Synthesis and secretion of a Bacillus circulans WL-12 1,3-1,4-beta-D-glucanase in Escherichia coli. J Bacteriol. 1990 Apr;172(4):2160–2167. doi: 10.1128/jb.172.4.2160-2167.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Cantwell B. A., McConnell D. J. Molecular cloning and expression of a Bacillus subtilis beta-glucanase gene in Escherichia coli. Gene. 1983 Aug;23(2):211–219. doi: 10.1016/0378-1119(83)90053-7. [DOI] [PubMed] [Google Scholar]
- Dierick N. A. Biotechnology aids to improve feed and feed digestion: enzymes and fermentation. Arch Tierernahr. 1989 Mar;39(3):241–261. doi: 10.1080/17450398909429530. [DOI] [PubMed] [Google Scholar]
- Gormley E. P., Cantwell B. A., Barker P. J., Gilmour R. S., McConnell D. J. Secretion and processing of the Bacillus subtilis endo-beta-1,3-1,4-glucanase in Escherichia coli. Mol Microbiol. 1988 Nov;2(6):813–819. doi: 10.1111/j.1365-2958.1988.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Gosalbes M. J., Pérez-González J. A., González R., Navarro A. Two beta-glycanase genes are clustered in Bacillus polymyxa: molecular cloning, expression, and sequence analysis of genes encoding a xylanase and an endo-beta-(1,3)-(1,4)-glucanase. J Bacteriol. 1991 Dec;173(23):7705–7710. doi: 10.1128/jb.173.23.7705-7710.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hecker M., Riethdorf S., Bauer C., Schroeter A., Borriss R. Expression of a cloned beta-glucanase gene from Bacillus amyloliquefaciens in an Escherichia coli relA strain after plasmid amplification. Mol Gen Genet. 1988 Dec;215(1):181–183. doi: 10.1007/BF00331323. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Losick R. Regulation of a promoter that is utilized by minor forms of RNA polymerase holoenzyme in Bacillus subtilis. J Mol Biol. 1986 Oct 20;191(4):615–624. doi: 10.1016/0022-2836(86)90449-3. [DOI] [PubMed] [Google Scholar]
- Janssen G. R., Bibb M. J. Derivatives of pUC18 that have BglII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene. 1993 Feb 14;124(1):133–134. doi: 10.1016/0378-1119(93)90774-w. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lloberas J., Perez-Pons J. A., Querol E. Molecular cloning, expression and nucleotide sequence of the endo-beta-1,3-1,4-D-glucanase gene from Bacillus licheniformis. Predictive structural analyses of the encoded polypeptide. Eur J Biochem. 1991 Apr 23;197(2):337–343. doi: 10.1111/j.1432-1033.1991.tb15916.x. [DOI] [PubMed] [Google Scholar]
- Louw M. E., Reid S. J., Watson T. G. Characterization, cloning and sequencing of a thermostable endo-(1,3-1,4) beta-glucanase-encoding gene from an alkalophilic Bacillus brevis. Appl Microbiol Biotechnol. 1993 Jan;38(4):507–513. doi: 10.1007/BF00242946. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Meissner P. S., Sisk W. P., Berman M. L. Bacteriophage lambda cloning system for the construction of directional cDNA libraries. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4171–4175. doi: 10.1073/pnas.84.12.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Olsen O., Borriss R., Simon O., Thomsen K. K. Hybrid Bacillus (1-3,1-4)-beta-glucanases: engineering thermostable enzymes by construction of hybrid genes. Mol Gen Genet. 1991 Feb;225(2):177–185. doi: 10.1007/BF00269845. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimming S., Schwarz W. H., Staudenbauer W. L. Structure of the Clostridium thermocellum gene licB and the encoded beta-1,3-1,4-glucanase. A catalytic region homologous to Bacillus lichenases joined to the reiterated domain of clostridial cellulases. Eur J Biochem. 1992 Feb 15;204(1):13–19. doi: 10.1111/j.1432-1033.1992.tb16600.x. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zverlov V. V., Laptev D. A., Tishkov V. I., Velikodvorskaja G. A. Nucleotide sequence of the Clostridium thermocellum laminarinase gene. Biochem Biophys Res Commun. 1991 Dec 16;181(2):507–512. doi: 10.1016/0006-291x(91)91217-z. [DOI] [PubMed] [Google Scholar]