Abstract
Aims
Sedation induced by antihistamines is widely recognized to be caused by their penetration through the blood–brain-barrier and the consequent occupation of brain histamine H1-receptors. We previously studied the mechanism of sedation caused by antihistamines using positron emission tomography (PET). Recently, we revealed the nonsedative characteristic of ebastine, a second-generation antihistamine, with cognitive performance tests. In the present study, H1-receptor occupation by ebastine was examined in the human brain using PET.
Methods
Ebastine 10 mg and (+)-chlorpheniramine 2 or 6 mg were orally given to healthy male volunteers. PET scans with [11C]-doxepin, a potent H1-receptor antagonist, were conducted near tmax of respective drugs. Other volunteers in the control group also received PET scans. The binding potential of doxepin (BP=Bmax/Kd) for available brain H1-receptors was imaged on a voxel-by-voxel basis through graphical analysis. By setting regions of interest, the H1-receptor occupancy of drugs was calculated in several H1-receptor rich regions.
Results
Brain distribution of radioactivity after ebastine treatment was similar to that without any drugs. However, after the oral administration of 2 mg (+)-chlorpheniramine, the level was lower than after ebastine and nondrug treatments. Graphical analysis followed by statistical parametric mapping (SPM96) revealed that H1-receptor rich regions such as cortices, cingulate gyrus and thalamus were regions where the BPs after ebastine were significantly higher than after (+)-chlorpheniramine (2 mg). H1-receptor occupancies in cortex were approximately 10% by ebastine and ≥50% by either dose of (+)-chlorpheniramine (95% confidence interval for difference in the mean receptor occupancies: 27%, 54% for 2 mg and 35%, 62% for 6 mg vs ebastine, respectively). Receptor occupancies increased with increasing plasma concentration of (+)-chlorpheniramine, but not with concentration of carebastine, an active metabolite of ebastine.
Conclusions
Ebastine (10 mg orally) causes brain histamine H1-receptor occupation of approximately 10%, consistent with its lower incidence of sedative effect, whereas (+)-chlorpheniramine occupied about 50% of brain H1-receptors even at a low but sedative dose of 2 mg; occupancy of (+)-chlorpheniramine was correlated with plasma (+)-chlorpheniramine concentration.
Keywords: (+)-chlorpheniramine, ebastine, histamine H1-receptor, positron emission tomography (PET), receptor occupancy
Introduction
Antihistamines are widely used for relief from allergic diseases such as urticaria and rhinitis [1]. They are generally classified into two categories, classical and second-generation agents, and their sedative characteristics are well-known in clinical and over-the-counter (OTC) medications. Sedation is caused by their penetration into CNS through the blood–brain barrier and the consequent occupation of histamine H1-receptors [2]. We examined the mechanism of (+)-chlorpheniramine-induced sedation by human positron emission tomography (PET) studies [3, 4]. We also determined the values for brain H1-receptor occupancies of several second-generation antihistamines which are believed to be nonsedating [3]. Consequently, we observed tendencies that the H1-receptor occupancies of the second-generation agents were relatively lower than those of the sedating antihistamines.
Ebastine, a second-generation antihistamine, is efficacious in allergic rhinitis [5–9], and urticaria [10, 11]. Its pharmacologically active metabolite, carebastine, is a carboxylic acid derivative formed by the oxidation at methyl-carbon on tert-butyl group of ebastine mainly by the first-pass effect [12, 13]. Carebastine is a polar metabolite of ebastine, suggesting that it is more difficult to penetrate the CNS than ebastine. In fact, the study using rats showed this phenomenon, in which the concentration ratio of brain to blood at 5 min after injection of [14C]-carebastine was lower than after [14C]-ebastine injection [14]. Recently, using attention-demanding cognitive tasks, we demonstrated that ebastine did not cause significant sedation [15]. In that study, comparing the effects of ebastine and (+)-chlorpheniramine on cognitive performance, we also revealed that the cognitive functions were not affected by the increase of plasma concentration of carebastine, but were impaired by that of (+)-chlorpheniramine concentration. In accordance with our data, the nonsedative characteristic of ebastine was demonstrated as one of self-reported adverse effects in the clinical phase III studies [9, 16].
In this study, we examined brain histamine H1-receptor occupancies of ebastine and (+)-chlorpheniramine orally in healthy men using PET, and compared the degrees of both receptor occupancies in order to characterize the mechanisms of their differential sedative effects. We also examined the relationship between their H1-receptor occupancies and the plasma drug concentrations.
Methods
This study was approved by the Committee on Clinical Investigation, Tohoku University School of Medicine (ethics committee), and was performed in accordance with the policy of the Declaration of Helsinki.
Subjects
Twenty-four healthy men, aged between 20 and 27 years old (average 22.8 ± 0.4 years), were enrolled in this study, and were classified into four groups as shown in Table 1. No subjects had a history of alcohol-dependency or any other drug-dependency or drug allergy. They were forbidden medication containing any antihistamines a week before the study, and were asked to abstain from any drugs and alcohol the night before the study and from tobacco, alcohol, caffeine, grapefruit, beverages including grapefruit and any other drugs during the test. Written informed consent was obtained from all subjects before the PET study.
Table 1.
Groups of subjects in this PET study
| Study | Group | Drug | Age (years) | Number of subjects | PET scanner | Study type |
|---|---|---|---|---|---|---|
| 1 | 1 | E | 24.0 ± 0.4 | 6 | SET 2400W | Single-blind, randomized and crossover study |
| C2 | ||||||
| 2 | ND | 24.3 ± 0.9 | 6 | |||
| 2 | 3 | C6 | 21.5 ± 0.2 | 6 | PT931 | Single-blind study |
| 4 | P | 21.5 ± 0.4 | 6 | |||
| Total | 22.8 ± 0.4 | 24 |
E: ebastine 10 mg, C2 and C6: (+)-chlorpheniramine 2 and 6 mg, ND: nondrug treatment, P: placebo.
Drug administration
Drugs used in the study were ebastine tablet (E) (10 mg), (+)-chlorpheniramine consisted of 2 mg (C2) and 6 mg (C6) (Repetabs) tablets for the positive controls and placebo tablet (P). Drugs were taken orally with approximately 150 ml of water.
PET measurement
Subjects were positioned in a SET2400W (Shimadzu Inc., Japan) or ECAT PT931/04–12 (CTI Inc, Knoxville, TN, USA) scanner, so that transaxial slices were parallel to the orbito-meatal line. The SET2400W scanner collects 63 simultaneous transverse slices with a spatial resolution of 4 mm (transaxial) and 4.5 mm (axial) full-width at half-maximum (FWHM) in the centre of the field of view (FOV) [17]. The ECAT PT931/04–12 scanner produces seven simultaneous transverse planes (four direct and three cross planes) with a spatial resolution of 8 mm (transaxial) and 7 mm (axial) FWHM in the centre of FOV [18]. Following a 68Ge/68Ga transmission scan, dynamic PET images were obtained for 90 min (sequential 22 scans: 6 scans × 90 s, 7 scans × 180 s, 6 scans × 300 s and 3 scans × 600 s) after an intravenous injection of [11C]-doxepin, which was synthesized as described previously [19]. The radiochemical and chemical purities of the ligand were more than 99% and more than 97%, respectively. The means±s.d. of the specific activity at the time of administration, injected dose and injected mass were 47.1 ± 18.6 GBq µmol−1 (1273 ± 503 mCi µmol−1), 427 ± 124 MBq (11.5 ± 3.34 mCi), and 9.0 ± 2.6 nmol (1.6 ± 0.5 µg), respectively.
Image analysis
Dynamic PET images were obtained in this study using the following image analyses. The averaged arterial blood concentration was used to calculate the values of the binding potential (BP=Bmax/Kd) of doxepin for available brain H1-receptors in each subject as reported previously [4, 20]. Parametric neuroimages which present the volume of distribution (Vd) for [11C]-doxepin were generated by graphical analysis [4, 21]. A region of interest (ROI) was placed on the cerebellum, as a reference region, in the neuroimages of Vd, and then neuroimages of BP were constructed by subtracting 1.0 from the Vd value in each voxel divided by the cerebellar ROI value according to the method described previously [21]. The parametric neuroimages of BP obtained by the SET2400W scanner were analysed statistically on a voxel-by-voxel basis by statistical parametric mapping (SPM96) software [22–24], in order to compare the bindings of ebastine and (+)-chlorpheniramine 2 mg on available brain H1-receptors. The images of the distributed radioactivity after injection of [11C]-doxepin were matched to the regional cerebral blood flow template which conformed to the standard anatomical space [25], and the estimated parameters for the spatial normalization were applied to normalizing each of the neuroimages of BP. Following the normalization, the images were smoothed by an isotropic Gaussian kernel with FWHM of 16 mm. Differences in the parameter values between ebastine and (+)-chlorpheniramine treatments were statistically analysed by the paired t-test (under multisubjects and different conditions) without any corrections for the global value. The SPM{t} was transformed to a SPM{Z}, and the distribution of Z-values was evaluated. Regional maxima of statistical significance (P < 0.05) were defined as voxels with higher Z-values than other voxels within 8 mm.
In addition to the analyses of the parametric neuroimages of BP, ROI-based analyses were conducted in order to evaluate brain H1-receptor occupancy. Values of BP were obtained from ROIs placed on cortices, anterior cingulate cortex and thalamus in the images. Each ROI was set using an initial PET image (0–45 min after [11C]-doxepin injection), which reflects an image of cerebral blood flow. In addition, H1-receptor occupancies (%) in these regions were calculated by subtracting the BP value of the drug-treated group divided by that of the control group from 1.0 and then expressing as a percentage. These values of BP and H1-receptor occupancy in each ROI were compared among the groups treated with ebastine and two doses of (+)-chlorpheniramine.
Study design
We designed a single-blind, randomized and crossover study in group 1 of ebastine 10 mg and (+)-chlorpheniramine 2 mg treatments, single-blind and randomized studies in group 3 and group 4 of (+)-chlorpheniramine 6 mg and placebo treatments, respectively, and a nondrug treatment study in group 2 (Table 1). The PET examinations of groups 2 and 4 were regarded to be the control for the studies of groups 1 and 3, respectively.
PET scans were started at around tmax of the respective antihistamines with 90 min scanning: namely, subjects were given ebastine 5 h and (+)-chlorpheniramine 2 h prior to PET scans [12, 26]. Subjects in group 1 were given two drugs randomly, and the respective experimental days were separated at an interval of at least 6 days. Placebo were given to subjects in group 4 2 h prior to the PET scans.
During the PET scans, blood was taken from subjects at various time points for analyses of plasma ebastine, carebastine and (+)-chlorpheniramine. Since the half-life of each drug is relatively long (ebastine 14–15 h and (+)-chlorpheniramine 12–15 h [12, 26]), the time period during the PET scans was assumed to be the time of the maximal plasma concentration. For further analyses, the respective mean plasma concentrations during PET scans were used as the representative of each PET scan.
Analysis of plasma drug concentration
Plasma concentrations of ebastine and carebastine were measured by a high performance liquid chromatography (h.p.l.c.) and of (+)-chlorpheniramine was measured by a liquid chromatography-mass spectrometry (LC-MS) at Dainippon Pharmaceutical Co., Ltd, as described previously [15].
Data analysis
The comparison between parametric neuroimages of BP after ebastine and (+)-chlorpheniramine 2 mg treatments were analysed by SPM96 under multisubjects and different conditions. Results in the ROI-based analysis are expressed as means±s.d. Following one-way anova, the Dunnett test was conducted for multiple comparisons of BP in Study 1 and H1-receptor occupancy among groups. BP resulting from Study 2 was analysed by the Student's t-test. The relationship between plasma drug concentration and H1-receptor occupancy was evaluated using the Spearman's rank correlation. A probability of less than 0.05 was considered to be statistically significant.
Apparent Kd values (dissociation equilibrium constant of (+)-chlorpheniramine) were estimated by analysis of the receptor occupancy and the plasma concentration using Michaelis–Menten model with the equation:
where [R] is brain H1-receptor occupancy, Rmax is the maximum receptor occupancy (regarded as 100% in this case), [Cp] is the plasma concentration of (+)-chlorpheniramine, and Kd is the dissociation equilibrium constant of (+)-chlorpheniramine for the H1-receptor.
Role of the study sponsor
The industry sponsor had a consulting role in the design, conduct, and reporting of the study. The authors from a pharmaceutical company only measured the plasma drug concentrations without noticing any precise data. Decisions in all aspects of the study, including the decision to publish the results, were made by the study group of Tohoku University.
Results
Distribution of [11C]-doxepin
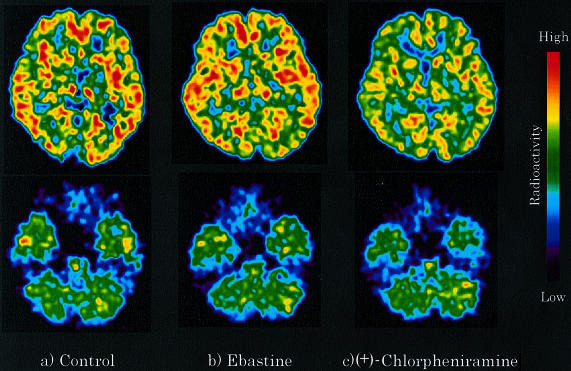
Representative PET images obtained during 45–90 min after injection of [11C]-doxepin at the striatal and cerebellar levels are shown in Figure 1. The distribution patterns of radioactivity in the ebastine-treated group were similar to those in the control (nondrug treatment) group. Namely, in both groups, high radioactivity was observed in the frontal, temporal and occipital cortices, cingulate gyrus, striatum and thalamus. In contrast to the images of the ebastine-treated or control group, the radioactivity in the (+)-chlorpheniramine 2 mg treated group was apparently lower in the regions mentioned above. The extent of binding of [11C]-doxepin to brain H1-receptors after ebastine treatment was virtually the same as that in the control group, while after the treatment of (+)-chlorpheniramine 2 mg, the binding was relatively low.
Figure 1.
Brain distribution of [11C]-doxepin radioactivity was examined in healthy male subjects by PET after the treatments of antihistamines. (a) Control (nondrug treatment), (b) ebastine 10 mg treatment, and (c) (+)-chlorpheniramine 2 mg treatment. Typical representatives of PET images are shown at the striatal and cerebellar levels. The images were obtained at 45–90 min after the injection of [11C]-doxepin.
Comparison of the parametric neuroimages of BP(ebastine vs (+)-chlorpheniramine 2 mg)
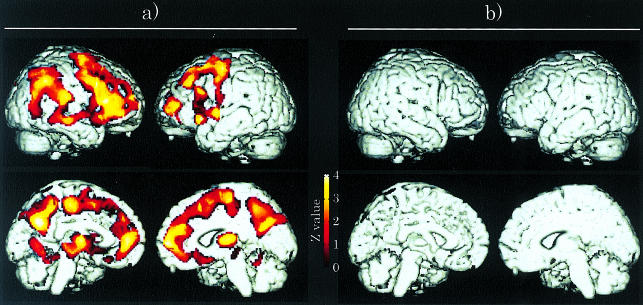
Parametric neuroimages of BP of doxepin after ebastine 10 mg and (+)-chlorpheniramine 2 mg treatments were constructed by graphical analysis and then were statistically compared with each other by SPM96 on a voxel-by-voxel basis. Figure 2a shows the coloured areas where the BP after ebastine treatment were significantly higher than those after (+)-chlorpheniramine 2 mg treatment. More H1-receptors were occupied by (+)-chlorpheniramine than by ebastine in these areas. These areas were the cortices, especially the frontal and prefrontal cortices, cingulate gyrus and thalamus, which are known to be the H1-receptor rich regions (Table 2). On the other hand, the SPM analyses could not detect any areas where the BPs after (+)-chlorpheniramine 2 mg treatment were significantly higher than those after ebastine treatment (Figure 2b).
Figure 2.
a) The coloured areas show that the BP of [14C]-doxepin after ebastine 10 mg treatment were significantly higher than those after (+)-chlorpheniramine 2 mg treatment (P < 0.05, uncorrected) using SPM96. This means that those areas show the higher H1-receptor occupation of (+)-chlorpheniramine than that of ebastine. In contrast, (b) there is no area showing that the BP after (+)-chlorpheniramine treatment was higher those after ebastine treatment.
Table 2.
Typical areas of higher brain H1-receptor occupancy in the (+)-chlorpheniramine 2 mg treatment compared with those in the ebastine treatment (P < 0.05, uncorrected)
| Area | Brodmann's area | x | y | z | Z-value |
|---|---|---|---|---|---|
| Frontal cortex | 6 | 28 | 16 | 52 | 4.74 |
| 6 | −12 | −24 | 48 | 4.45 | |
| Prefrontal cortex | 10 | −38 | 52 | 10 | 3.68 |
| 8 | −34 | 60 | 38 | 3.45 | |
| Posterior cingulate cortex | 31 | 0 | −60 | 26 | 3.43 |
| Supramarginal gyrus | 40 | 48 | −48 | 36 | 3.27 |
| Thalamus | 16 | −20 | 12 | 3.19 |
ROI-based comparisons of BPs and H1-receptor occupancies
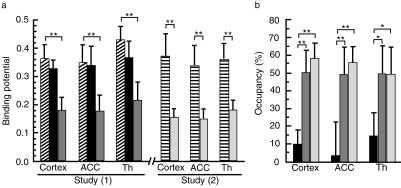
The BP values in H1-receptor rich regions such as the cortex, anterior cingulate cortex (ACC) and thalamus were evaluated after the administration of ebastine and two doses of (+)-chlorpheniramine (2 and 6 mg) with the ROI-based analyses. In study 1, the BP values of all regions in the control (nondrug-treated) and ebastine-treated groups showed no difference while those in the (+)-chlorpheniramine 2 mg treated group were significantly lower than those in the control group as shown in Figure 3a (cortex: P < 0.001, 95% confidence intervals for difference in the mean BPs: 0.125, 0.242, ACC: P < 0.001, 95% CI: 0.086, 0.259 and thalamus: P < 0.001, 95% CI: 0.134, 0.294). In study 2, the BP values of all the regions in the (+)-chlorpheniramine 6 mg treated group were also significantly lower than those in the control (placebo-treated) group (cortex: P < 0.001, 95% CI: 0.142, 0.293, ACC: P < 0.001, 95% CI: 0.168, 0.224 and thalamus: P < 0.001, 95% CI: 0.171, 0.228).
Figure 3.
ROI-based analyses of BPs and H1-receptor occupancies in the cortex, anterior cingulate cortex (ACC) and thalamus (Th) after antihistamine treatments. (a) In study 1, the comparisons of BPs are shown among the control ( ), ebastine (▪) and (+)-chlorpheniramine 2 mg (
), ebastine (▪) and (+)-chlorpheniramine 2 mg ( ) groups. In study 2, the BP values are compared between the placebo (
) groups. In study 2, the BP values are compared between the placebo ( ) and (+)-chlorpheniramine 6 mg (
) and (+)-chlorpheniramine 6 mg ( ) treatment groups. (b) H1-receptor occupancies by antihistamines (ebastine ▪; (+)-chlorpheniramine 2 mg
) treatment groups. (b) H1-receptor occupancies by antihistamines (ebastine ▪; (+)-chlorpheniramine 2 mg  ; and (+)-chlorpheniramine 6 mg
; and (+)-chlorpheniramine 6 mg  ) are shown when the occupancy in the control or placebo group is regarded as 0%. *P< 0.01 and **P< 0.001, statistically analysed by Dunnett's multiple comparison test or by Student's t-test.
) are shown when the occupancy in the control or placebo group is regarded as 0%. *P< 0.01 and **P< 0.001, statistically analysed by Dunnett's multiple comparison test or by Student's t-test.
The H1-receptor occupancies after ebastine and (+)-chlorpheniramine treatments were calculated in the cortex, ACC and thalamus, when the respective occupancies in the control groups were regarded as 0% (Figure 3b). The respective H1-receptor occupancies were calculated to be approximately 9.9, 3.2 and 14.4% in the ebastine-treated group, approximately 50.3 (P < 0.001, 95% CI for difference in the mean receptor occupancies: 26.6, 54.3 vs ebastine), 49.2 (P < 0.001, 95% CI: 24.3, 67.5 vs ebastine) and 49.7% (P < 0.01, 95% CI: 14.8, 55.9 vs ebastine) in the (+)-chlorpheniramine 2 mg-treated group, and approximately 58.3 (P < 0.001, 95% CI: 34.6, 62.2 vs ebastine), 55.9 (P < 0.001, 95% CI: 31.1, 74.3 vs ebastine) and 49.6% (P < 0.01, 95% CI: 14.6, 55.8 vs ebastine) in the (+)-chlorpheniramine 6 mg treated group. These data demonstrate that the H1-receptor occupancies by ebastine are substantially lower than those following either of the two doses of (+)-chlorpheniramine in all regions.
Relationship between H1-receptor occupancy and plasma drug concentration
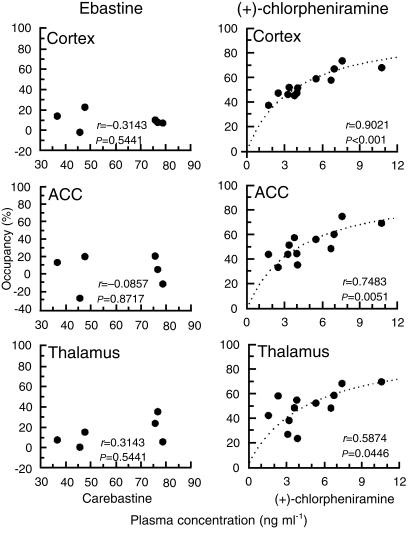
The relationships between the H1-receptor occupancies in the cortex, ACC and thalamus, and plasma concentration of carebastine or (+)-chlorpheniramine are shown in Figure 4. In this figure, the mean plasma concentrations during PET scans were used. In the ebastine-treated group, the H1-receptor occupancies were not correlated with the plasma concentration of carebastine in any of the regions. In contrast, the receptor occupancies in the cortex, ACC and thalamus increased significantly along with the plasma concentration of (+)-chlorpheniramine; [cortex: r=0.9021 (P < 0.001); ACC: r=0.7483 (P = 0.0051); thalamus: r=0.5874 (P = 0.0446)].
Figure 4.
Relationships between H1-receptor occupancies in the cortex, anterior cingulate cortex (ACC) and thalamus and plasma drug concentration. The x-axis of the ebastine group is plasma carebastine concentration. Correlations were statistically analysed by Spearman's rank correlation test (P < 0.05: statistical significant). Dotted curves reflect the estimated curves of relationships between plasma concentration and the receptor occupancy analysed by the Michaelis–Menten equation.
Discussion
In the present study, we investigated the brain H1-receptor occupancies of ebastine and (+)-chlorpheniramine in healthy men using PET with [11C]-doxepin. This study revealed that brain H1-receptor binding of doxepin did not significantly change after ebastine treatment when compared with the control. However, doxepin binding decreased significantly following (+)-chlorpheniramine treatment in H1-receptor rich regions such as cortices, cingulate gyrus and thalamus. Two different approaches of imaging analysis (ROI-based analysis and voxel-by-voxel examination using SPM96) gave similar results.
In addition to the voxel-by-voxel analysis, H1-receptor occupancies by the antihistamines were accessed using the ROI-based analysis assuming that H1-receptor occupancies in the control group were 0%. The occupancies of ebastine in the regions such as the cortex, ACC and thalamus were calculated to be approximately 10%. These values were significantly lower than the corresponding values of ≥50% following either dose of (+)-chlorpheniramine. Our previous studies demonstrated that several second-generation antihistamines such as epinastine, terfenadine, azelastine, mequitazine and astemizole occupy 10–30% of brain H1-receptors [3]. We recently demonstrated that ebastine does not impair cognitive functions nor induce sleepiness in healthy normal subjects [15]. Those studies together with our present study lead to a recognition of the nonsedative characteristic of ebastine due to lower brain H1-receptor occupancy.
In contrast to ebastine, the H1-receptor occupancies of (+)-chlorpheniramine 2 and 6 mg were (≤50% in all regions) analysed. Our previous study revealed that impairment of cognitive performance and sleepiness occurred following (+)-chlorpheniramine 2 mg [15]. Our present and previous studies demonstrate that cognitive function and brain H1-receptor occupancy by (+)-chlorpheniramine are significantly correlated with the plasma concentration of (+)-chlorpheniramine. These data support the conclusion that the impaired cognitive function and subjective sleepiness induced by (+)-chlorpheniramine are caused by H1-receptor occupation [4], and that H1-receptor occupancy of ≥50% impairs cognitive performance.
Dotted curves shown in Figure 4 were fitted by analysis of H1-receptor occupancy and the plasma concentration using Michaelis–Menten model (assuming the maximum of H1-receptor occupancy as 100%). Consequently, apparent Kd values (dissociation equilibrium constant) of (+)-chlorpheniramine for H1-recptors in the cortex, ACC and thalamus were calculated to be 6.43 (95% CI: 5.55, 7.31), 6.98 (95% CI: 5.29, 8.67) and 7.97 (95% CI: 5.24, 10.70) nm, respectively, based on free unbound plasma concentration of (+)-chlorpheniramine assuming that its plasma protein binding was 32% [27]. Since the free drug concentration in plasma is equal to that in the tissue (brain), the calculated mean Kd value of about 7 nm could be the intrinsic Kd value of (+)-chlorpheniramine for brain H1-receptors. In fact, the Kd value is virtually in the same order as those determined in vitro of 4.0 nm in the human prefrontal cortex and of 3.0 nm in HeLa cells [28, 29]. Thus, using the fitted curves, the brain H1-receptor occupancy of (+)-chlorpheniramine can be predicted from its plasma concentration.
There is no evidence for different subtypes of CNS and peripheral H1-receptors from bovine or guinea pig studies [30, 31]. H1-receptors are absent in both central nervous and peripheral tissues of H1-receptor-gene knocked-out animals [32]. Moreover, a second-generation antihistamine, terfenadine has a high affinity for central H1-receptors in in vitro conditions [33]. Drugs with high affinity for peripheral H1-receptors can bind to brain H1-receptors, provided they gain access to them. Second-generation antihistamines induce sedation if they are transported into the brain to occupy its H1-receptors [34]. On the other hand, ebastine does not occupy brain H1-receptors in parallel with increasing plasma carebastine concentration, perhaps, because carebastine is a substrate of P-glycoprotein and other transporters expressed on the blood–brain-barrier, which serve as efflux pumps from the brain to the blood [35]. Using the BUI (brain uptake index) method in rats, the efflux of [14C]-carebastine by the transporters was not inhibited by a large amount of nonlabelled carebastine (150 µm) [35], which was about 650 times the plasma concentration obtained from the clinical phase I study [12]. These facts suggest that ebastine causes little sedation even when associated with a high plasma carebastine concentration as a result of overdosing or metabolic inhibition.
In conclusion, ebastine occupied only approximately 10% of available H1-receptors in human brain. On the other hand, approximately 50% of the H1-receptors was occupied by (+)-chlorpheniramine even at a low dose of 2 mg. The low H1-receptor occupancy by ebastine is thought to result in the nonsedative characteristics of this agent. On the other hand, the higher H1-receptor occupation caused the sedative properties of (+)-chlorpheniramine. This study also demonstrates the possibility of predicting H1-receptor occupancy by (+)-chlorpheniramine from its plasma concentration.
Acknowledgments
This work was supported by grants-in-aid from the Ministry of Education, Science, Sports and Culture, Japan, the Ministry of Health and Welfare, Japan, and the Shimadzu Science Foundation. We thank Prof R. Kato (Keio University School of Medicine) for his encouragement of this study and Dr I. Sato (Miyagi Cancer Center) for his suggestion of statistical evaluation. We also appreciate the technical assistance of S. Watanuki, Y. Ishikawa, Y. Funaki, and M. Miyake in our PET studies.
References
- 1.Simons FE, Simons KJ. The pharmacology and use of H1-receptor-antagonist drugs. N Engl J Med. 1994;330:1663–1670. doi: 10.1056/NEJM199406093302307. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg MJ, Spector R, Chiang CK. Transport of diphenhydramine in the central nervous system. J Pharmacol Exp Ther. 1987;240:717–722. [PubMed] [Google Scholar]
- 3.Yanai K, Okamura N, Tagawa M, Itoh M, Watanabe T. New findings in pharmacological effects induced by antihistamines: from PET studies to knock-out mice. Clin Exp Allergy. 1999;29(Suppl 3):29–36. [PubMed] [Google Scholar]
- 4.Okamura N, Yanai K, Higuchi M, et al. Functional neuroimaging of cognition impaired by a classical antihistamine, d-chlorpheniramine. Br J Pharmacol. 2000;129:115–123. doi: 10.1038/sj.bjp.0702994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luria X. Comparative clinical studies with ebastine, efficacy and tolerability. Drug Saf. 1999;21(Suppl 1):63–67. doi: 10.2165/00002018-199921001-00008. [DOI] [PubMed] [Google Scholar]
- 6.de Molina M, Cadahia L, Cano L, et al. Efficacy and tolerability of ebastine at two dose levels in the treatment of seasonal allergic rhinitis. Drug Invest. 1989;1:40–46. [Google Scholar]
- 7.Gehannol P, Brémard-Oury C, Zeisser P. A double-blind multicenter randomized study comparing once daily oral administration of ebastine 20 mg, ebastine 10 mg, and cetirizine 10 mg for the treatment of seasonal allergic rhinitis in adults. Rhône-Poulenc Rorer (Collegeville). (Data on file)
- 8.Picado VC, Cadahia GA, Cistero BA, Cano CL, Sanz AA, Zayas SJM. Ebastine in perennial allergic rhinitis. Ann Allergy. 1991;67:615–618. [PubMed] [Google Scholar]
- 9.Baba S, Mamiya S, Sakakura Y, et al. Clinical trial of LAS-90 on perennial allergic rhinitis: a double blind study in comparison with ketotifen fumarate [in Japanese] Rinsho Iyaku. 1994;10:1143–1162. [Google Scholar]
- 10.Kalis B, Brémard-Oury C. A 3-month double-blind comparative study of ebastine (10 mg o.d.), terfenadine (60 mg b.i.d.) and placebo in the treatment of chronic urticaria. Allergy. 1995;50(Suppl):380. [Google Scholar]
- 11.Peyri J, Vidal J, Marrón J, et al. Ebastine in chronic urticaria: a double-blind placebo-controlled study. J Dermatol Treat. 1991;2:51–53. [Google Scholar]
- 12.Yamaguchi T, Hashizume T, Matsuda M, et al. Pharmacokinetics of the H1-receptor antagonist ebastine and its active metabolite carebastine in healthy subjects. Arzneimittelforschung Drug Res. 1994;44:59–64. [PubMed] [Google Scholar]
- 13.Vincent J, Liminana R, Meredith PA, Reid JL. The pharmacokinetics, antihistamine and concentration-effect relationship of ebastine in healthy subjects. Br J Clin Pharmacol. 1988;26:497–502. doi: 10.1111/j.1365-2125.1988.tb05288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii T, Matsumoto S, Amejima H, et al. Absorption, distribution, metabolism and excretion of [14C]ebastine after a single administration in rats. Arzneimittelforschung Drug Res. 1994;44:527–538. [PubMed] [Google Scholar]
- 15.Tagawa M, Kano M, Okamura N, et al. Differential cognitive effects of ebastine and (+)-chlorpheniramine in healthy subjects: Correlation between cognitive impairment and plasma drug concentration. Br J Clin Pharmacol. (in press) [DOI] [PMC free article] [PubMed]
- 16.Kukita A, Harada S, Yoshida H, et al. Phase III study of LAS-90 on chronic urticaria: double blind comparative study with ketotifen fumarate [in Japanese] Rinsho Iyaku. 1994;10:895–912. [Google Scholar]
- 17.Fujiwara T, Watanuki S, Yamamoto S, et al. Performance evaluation of a large axial field-of-view PET scanner SET-2400W. Ann Nucl Med. 1997;11:307–313. doi: 10.1007/BF03165298. [DOI] [PubMed] [Google Scholar]
- 18.Spinks TJ, Guzzardi R, Bellina CR. Performance characteristics of a whole body positron tomograph. J Nucl Med. 1988;29:1833–1841. [PubMed] [Google Scholar]
- 19.Yanai K, Watanabe T, Yokoyama H, et al. Histamine H1 receptors in human brain visualized in vivo by [11C]doxepin and positron emission tomography. Neurosci Lett. 1992;137:145–148. doi: 10.1016/0304-3940(92)90390-s. [DOI] [PubMed] [Google Scholar]
- 20.Yanai K, Ryu JH, Watanabe T, et al. Histamine H1 receptor occupancy in human brains after single oral doses of histamine H1 antagonists measured by positron emission tomography. Br J Pharmacol. 1995;116:1649–1655. doi: 10.1111/j.1476-5381.1995.tb16386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan J, Fowler JS, Volkow ND, et al. Graphical analysis of reversible radioligand binding form time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 22.Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- 23.Higuchi M, Yanai K, Okamura N, et al. Histamine H1 receptors in patients with Alzheimer's disease assessed by positron emission tomography (PET) Neuroscience. 2000;99:721–729. doi: 10.1016/s0306-4522(00)00230-x. [DOI] [PubMed] [Google Scholar]
- 24.Higuchi M, Itoh M, Yanai K, et al. Brain mapping of the effects of aging on histamine H1 receptors in humans: A PET study with [11C]-doxepin. In: Carson R, Daube-Witherspoon M, Herscovitch P, editors. Quantitative functional brain imaging with positron emission tomography. San Diego: Academic Press; 1998. pp. 207–214. Chapter 31. [Google Scholar]
- 25.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme. Medical Publishers, Inc.; 1988. [Google Scholar]
- 26.Peets EA, Jackson M, Symchowicz S. Metabolism of chlorpheniramine maleate in man. J Pharmacol Exp Ther. 1972;180:464–474. [PubMed] [Google Scholar]
- 27.Hiep BT, Gimenez F, Khanh VU, et al. Binding of chlorpheniramine enantiomers to human plasma proteins. Chirality. 1999;11:501–504. doi: 10.1002/(SICI)1520-636X(1999)11:5/6<501::AID-CHIR24>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 28.Chang RS, Tran VT, Snyder SH. Heterogeneity of histamine H1-receptors: species variations in [3H]mepyramine binding of brain membranes. J Neurochem. 1979;32:1653–1663. doi: 10.1111/j.1471-4159.1979.tb02276.x. [DOI] [PubMed] [Google Scholar]
- 29.Arias-Montano JA, Young JM. Characteristics of histamine H1 receptors on HeLa cells. Eur J Pharmacol. 1993;245:291–295. doi: 10.1016/0922-4106(93)90110-u. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita M, Fukui H, Sugama K, et al. Expression cloning of a cDNA encoding the bovine histamine H1 receptor. Proc Natl Acad Sci USA. 1991;88:11515–11519. doi: 10.1073/pnas.88.24.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ter Laak AM, Donne-Op den Kelder GM, Bast A, Timmerman H. Is there a difference in the affinity of histamine H1 receptor antagonists for CNS and peripheral receptors? An in vitro study. Eur J Pharmacol. 1993;232:199–205. doi: 10.1016/0014-2999(93)90774-c. [DOI] [PubMed] [Google Scholar]
- 32.Inoue I, Yanai K, Kitamura D, et al. Impaired locomotor activity and exploratory behaviour in mice lacking histamine H1 receptors. Proc Natl Acad Sci USA. 1996;93:13316–13320. doi: 10.1073/pnas.93.23.13316. 10.1073/pnas.93.23.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose C, Quach TT, Llorens C, Schwartz JC. Relationship between occupation of cerebral H1-receptors and sedative properties of antihistamines. Assessment in the case of terfenadine. Arzneim-Forsch/Drug Res. 1982;32:1171–1173. [PubMed] [Google Scholar]
- 34.Yanai K, Ryu JH, Watanabe T, et al. Positron emission tomographic study of central histamine H1-receptor occupancy in human subjects treated with epinastine, a second-generation antihistamine. Meth Find Exp Clin Pharmacol. 1995;17(Suppl C):64–69. [PubMed] [Google Scholar]
- 35.Tamai I, Kido Y, Yamashita J, Sai Y, Tsuji A. Blood–brain barrier transport of H1-antagonist ebastine and its metabolite carebastine. J Drug Targeting. 2000;8:383–393. doi: 10.3109/10611860008997914. [DOI] [PubMed] [Google Scholar]