Abstract
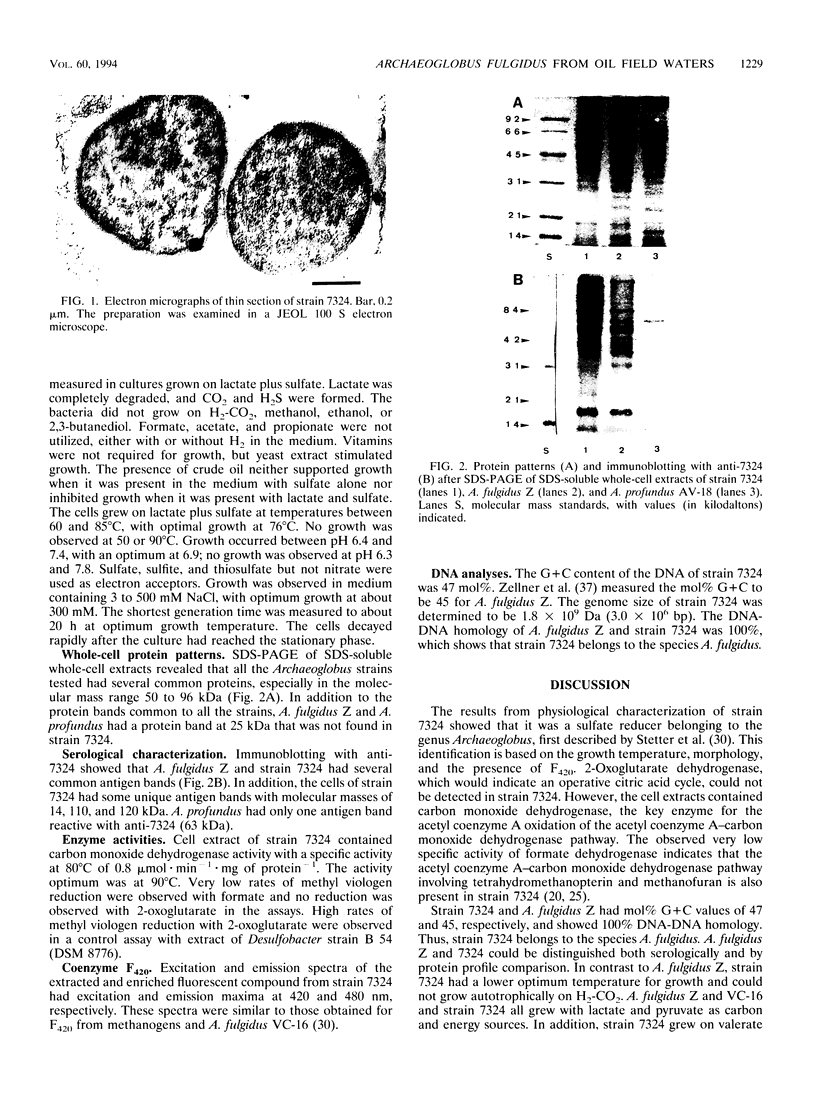
A hyperthermophilic sulfate reducer, strain 7324, was isolated from hot (75°C) oil field waters from an oil production platform in the Norwegian sector of the North Sea. It was enriched on a complex medium and isolated on lactate with sulfate. The cells were nonmotile, irregular coccoid to disc shaped, and 0.3 to 1.0 μm wide. The temperature for growth was between 60 and 85°C with an optimum of 76°C. Lactate, pyruvate, and valerate plus H2 were utilized as carbon and energy sources with sulfate as electron acceptor. Lactate was completely oxidized to CO2. The cells contained an active carbon monoxide dehydrogenase but no 2-oxoglutarate dehydrogenase activity, indicating that lactate was oxidized to CO2 via the acetyl coenzyme A/carbon monoxide dehydrogenase pathway. The cells produced small amounts of methane simultaneously with sulfate reduction. F420 was detected in the cells which showed a blue-green fluorescence at 420 nm. On the basis of morphological, physiological, and serological features, the isolate was classified as an Archaeoglobus sp. Strain 7324 showed 100% DNA-DNA homology with A. fulgidus Z, indicating that it belongs to the species A. fulgidus. Archaeoglobus sp. has been selectively enriched and immunomagnetically captured from oil field waters from three different platforms in the North Sea. Our results show that strain 7324 may grow in oil reservoirs at 70 to 85°C and contribute to hydrogen sulfide formation in this environment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Christensen B., Torsvik T., Lien T. Immunomagnetically captured thermophilic sulfate-reducing bacteria from north sea oil field waters. Appl Environ Microbiol. 1992 Apr;58(4):1244–1248. doi: 10.1128/aem.58.4.1244-1248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypionka H. Characterization of sulfate transport in Desulfovibrio desulfuricans. Arch Microbiol. 1989;152(3):237–243. doi: 10.1007/BF00409657. [DOI] [PubMed] [Google Scholar]
- Diekert G. B., Thauer R. K. Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J Bacteriol. 1978 Nov;136(2):597–606. doi: 10.1128/jb.136.2.597-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. A. Sulphate-reducing bacteria and anaerobic corrosion. Annu Rev Microbiol. 1985;39:195–217. doi: 10.1146/annurev.mi.39.100185.001211. [DOI] [PubMed] [Google Scholar]
- Klein A. R., Breitung J., Linder D., Stetter K. O., Thauer R. K. N5,N10-methenyltetrahydromethanopterin cyclohydrolase from the extremely thermophilic sulfate reducing Archaeoglobus fulgidus: comparison of its properties with those of the cyclohydrolase from the extremely thermophilic Methanopyrus kandleri. Arch Microbiol. 1993;159(3):213–219. doi: 10.1007/BF00248474. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandel M., Igambi L., Bergendahl J., Dodson M. L., Jr, Scheltgen E. Correlation of melting temperature and cesium chloride buoyant density of bacterial deoxyribonucleic acid. J Bacteriol. 1970 Feb;101(2):333–338. doi: 10.1128/jb.101.2.333-338.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosnes J. T., Torsvik T., Lien T. Spore-forming thermophilic sulfate-reducing bacteria isolated from north sea oil field waters. Appl Environ Microbiol. 1991 Aug;57(8):2302–2307. doi: 10.1128/aem.57.8.2302-2307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwörer B., Breitung J., Klein A. R., Stetter K. O., Thauer R. K. Formylmethanofuran: tetrahydromethanopterin formyltransferase and N5,N10-methylenetetrahydromethanopterin dehydrogenase from the sulfate-reducing Archaeoglobus fulgidus: similarities with the enzymes from methanogenic Archaea. Arch Microbiol. 1993;159(3):225–232. doi: 10.1007/BF00248476. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stetter K. O., Lauerer G., Thomm M., Neuner A. Isolation of extremely thermophilic sulfate reducers: evidence for a novel branch of archaebacteria. Science. 1987 May 15;236(4803):822–824. doi: 10.1126/science.236.4803.822. [DOI] [PubMed] [Google Scholar]
- Torsvik V., Goksøyr J., Daae F. L. High diversity in DNA of soil bacteria. Appl Environ Microbiol. 1990 Mar;56(3):782–787. doi: 10.1128/aem.56.3.782-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Woese C. R., Achenbach L., Rouviere P., Mandelco L. Archaeal phylogeny: reexamination of the phylogenetic position of Archaeoglobus fulgidus in light of certain composition-induced artifacts. Syst Appl Microbiol. 1991;14(4):364–371. doi: 10.1016/s0723-2020(11)80311-5. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]




