Abstract
Background and purpose:
The acetylcholinesterase inhibitor, donepezil, is also a high affinity σ1 receptor agonist. We examined the involvement of σ1 receptors in its anti-amnesic and neuroprotective properties against amyloid β25-35 peptide-induced toxicity in mice.
Experimental approach:
Mice were given an intracerebroventricular (i.c.v.) injection of Aβ25-35 peptide (9 nmol) 7-9 days before being tested for spontaneous alternation and passive avoidance. Hippocampal lipid peroxidation was measured 7 days after Aβ25-35 injection to evaluate oxidative stress. Donepezil, the σ1 agonist PRE-084 or the cholinesterase (ChE) inhibitors tacrine, rivastigmine and galantamine were administered either 20 min before behavioural sessions to check their anti-amnesic effects, or 20 min before Aβ25-35 injection, or 24 h after Aβ25-35 injection and then once daily before behavioural sessions, to check their pre- and post-i.c.v. neuroprotective activity, respectively.
Key results:
All the drugs tested were anti-amnesic, but only the effects of PRE-084 and donepezil were prevented by the σ1 antagonist BD1047. Only PRE-084 and donepezil showed neuroprotection when administered pre i.c.v.; they blocked lipid peroxidation and learning deficits, effects inhibited by BD1047. Post i.c.v., PRE-084 and donepezil showed complete neuroprotection whereas the other ChE inhibitors showed partial effects. BD1047 blocked these effects of PRE-084, attenuated those of donepezil, but did not affect the partial effects of the other ChE inhibitors.
Conclusions and implications.
The potent anti-amnesic and neuroprotective effects of donepezil against Aβ25-35-induced toxicity involve both its cholinergic and σ1 agonistic properties. This dual action may explain its sustained activity compared to other ChE inhibitors.
Keywords: Donepezil, PRE-084, σ1 receptor, cholinesterase inhibitor, amyloid β25–35 peptide, amnesia, neuroprotection, lipid peroxidation
Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by a progressive loss of cognitive functions, leading to dementia and death. One of the main physiopathological features characterizing the disease is the presence of extracellular senile plaques, constituted mainly by accumulation of amyloid β (Aβ) proteins (Selkoe, 1991, 1994). Aβ proteins are generated from amyloid precursor protein (APP) by proteolytic cleavage and the main endogenous forms contain 40, 42 and 43 amino acids (Selkoe, 1991, 1994). Direct application of Aβ into primary neuronal cell cultures and other cell lines is highly toxic (Cotman and Anderson, 1995). Although the mechanism of the amyloid toxicity remains to be elucidated, its effects are dependent on the ability of the protein to aggregate into fibrillar amorphous structures (Pike et al., 1993). Structure–activity studies using Aβ fragments revealed that the peptide bearing the 11 amino acids (25–35) retains the ability to self-aggregate and mediate the toxicity in vitro (Malouf, 1992; Mattson et al., 1992; Pike et al., 1995) and in vivo (Maurice et al., 1996). This toxicity involves oxidative stress induction by production of free radicals (Behl et al., 1992; Cafe et al., 1996; Pike et al., 1997), disruption of calcium homeostasis (Mattson et al., 1992, 1993), enhancement of excitotoxicity (Mattson et al., 1992) and apoptosis (Forloni et al., 1993). Indeed, the intracerebroventricular (i.c.v.) administration of Aβ25–35 peptide into the rodent brain induced, within 1 or 2 weeks after administration, histological and biochemical changes, memory deficits (Flood et al., 1991; Kowall et al., 1991, 1992; Maurice et al., 1996) and oxidative stress (Stepanichev et al., 1998).
Cholinergic systems are very sensitive to amyloid toxicity and a well-characterized impairment of cholinergic neurons, within the nucleus basalis magnocellularis, or nucleus of Meynert in human, is involved in the rapid loss of learning and memory (Perry et al., 1978; Erme et al., 1992). Present strategies used to treat AD aim to improve or at least maintain central cholinergic functions, especially those involving cholinesterase (ChE) inhibitors. This allows a symptomatic alleviation of the cognitive deficits, but also putatively produces effective neuroprotection. Indeed, experimental evidence has shown that nicotinic receptor agonists are able to attenuate Aβ and glutamate toxicity in cultured neurons (Kihara et al., 1997; Zamani et al., 1997). Several ChE inhibitors, including tacrine, donepezil and galantamine, also attenuate Aβ1–40- or Aβ25–35-induced toxicity (Svensson and Nordberg, 1998; Arias et al., 2004; Kihara et al., 2004). This effect has been shown to involve an interaction with nicotinic receptors (Svensson and Nordberg, 1998) and more precisely the α7-nicotinic receptor subtype mediating activation of phosphatidylinositol 3-kinase (PI3K) (Kihara et al., 2004).
The different ChE inhibitors available at present exhibit different pharmacological profiles. In particular, whereas tacrine and rivastigmine inhibit acetylcholinesterase (AChE) and butyrylcholinesterase activities, donepezil is highly selective for AChE and galantamine acts as a weak ChE inhibitor (Ogura et al., 2000). Donepezil also interacts, within the same concentration range, with the σ1 receptor (Kato et al., 1999), and this may contribute to its symptomatic and neuroprotective effects (Maurice et al., 2006; Meunier et al., 2006). The σ1 receptor is an intracellular protein localized in the vicinity of the endoplasmic reticulum (ER). Its activation rapidly modulates the mobilization of inositol-1,2,4 trisphosphate receptor-gated calcium pools from the intracellular ER pools (Hayashi et al., 2000). This is of particular interest as Aβ toxicity has been shown to involve ER stress. Aβ proteins, and particularly Aβ25–35 peptide, induced disturbances of the ER homeostasis and activation of stress-responsive genes, such as grp 78 or grp 94 (Yu et al., 1999; Ghribi et al., 2004). These genes are known to act as molecular chaperons regulating protein folding and translocation into the ER and protein secretion (Lee, 1992). Moreover, σ1 receptor activation provokes its translocation, associated within lipid droplets to cholesterol and anchor proteins, from the ER towards plasma, mitochondria or nucleus membranes (Hayashi and Su, 2003). The σ1 receptors may play a role in the compartmentalization and export of lipids to peripheries of cells (Hayashi and Su, 2003, 2005; Takebayashi et al., 2004). Lipid rafts have a role in a variety of cellular functions including vesicle transport, receptor clustering, internalization and coupling of receptors with the proteins involved in signal transduction (Simons and Ikonen, 1997). Activation of σ1 receptors may, therefore, induce important effects on cell viability, differentiation and neuroprotection. Indeed, selective σ1 receptor agonists are potent neuroprotective drugs, as observed in excitotoxicity models (for a review, see Maurice and Lockhart, 1997; Nakazawa et al., 1998) and recently against Aβ25–35-induced toxicity in cortical neurons in vitro (Marrazzo et al., 2005).
In the present study, we examined the anti-amnesic and neuroprotective effects of donepezil, in comparison with the selective σ1 receptor agonist PRE-084 and other ChE inhibitors, tacrine, rivastigmine and galantamine, against Aβ-induced toxicity in vivo in mice. Animals were administered aggregated Aβ25–35 peptide, i.c.v., and the learning and memory impairments were checked after 1 week, using the spontaneous alternation and passive avoidance procedures. The level of lipid peroxidation, an index of oxidative stress, was measured in the hippocampus. Drugs were administered either 20 min before the behavioural procedures, that is, 1 week after Aβ25–35, to examine the anti-amnesic effects; or 20 min before Aβ25–35, that is, 1 week before the behavioural and biochemical measures, to examine the pre-i.c.v. protection; or 24 h after Aβ25–35 and once-a-day for 1 week before the behavioural and biochemical measures, to examine the post-i.c.v. protection. In addition, the involvement of the σ1 receptor in the pharmacological effects of the drugs was determined by pretreating the mice with the σ1 receptor antagonist BD1047.
Methods
Animals
A total of 944 male Swiss mice, 1-month old and weighing 28–32 g, were used. They were purchased from the breeding centre of the Faculty of Pharmacy (Montpellier, France) and then kept in the animal facility building of the University of Montpellier II. Animals were housed in groups of 20 with access to food and water ad libitum, except during the experiments. They were kept in a temperature and humidity-controlled animal facility on a 12 h/12 h light/dark cycle (lights off at 1900 hours). Behavioral experiments were carried out between 0900 and 1400 hours, in a soundproof and air-regulated experimental room, to which mice were habituated to for at least 30 min. All animal procedures were conducted in strict adherence to the European Communities Council Directive of 24 November 1986 (86–609).
Experimental series
Initially, the amnesic effects of aggregated Aβ25–35 peptide, administered i.c.v., were checked. Animals were administered increasing doses of Aβ25–35 or scrambled Aβ25–35 (i.c.v.) and learning and memory impairments were examined after 7 days. Then, as depicted in Figure 1, the anti-amnesic effects of the AChE inhibitors (donepezil, tacrine, rivastigmine, galantamine) or σ1 receptor agonist (PRE-084) were examined by pre-test injections, 7–8 days after Aβ25–35 administration. The neuroprotective effects of each compound were examined using two protocols: (i) injecting the compound 20 min before Aβ25–35 administration, animals being tested after 7–9 days (pre-i.c.v. protection) or (ii) injections given 1 h after Aβ25–35 administration and repeatedly once a day for 6–7 days, animals being tested on day 7–9 (post-i.c.v. protection).
Figure 1.
Experimental procedures. Animals were administered i.c.v. with amyloid Aβ25–35 peptide and examined for learning abilities after 7–9 days. Some animals were killed on day 7 for lipid peroxidation measurement in the hippocampus. Three drug administration schedules were used: (1) the anti-amnesic effects were tested by injecting drugs 20 min before behavioural testing, that is, 7–8 days after Aβ25–35 peptide; (2) the pre-i.c.v. neuroprotection was tested by injecting the drugs 20 min before Aβ25–35-peptide, that is, 7–8 days before behavioural testing; (3) the post-i.c.v. neuroprotection was tested by injecting the drugs 24 h after Aβ25–35 peptide and once a day for 6–7 days, with the last injection at least 20 h before behavioural testing.
Spontaneous alternation performances
The spatial working memory was examined by measuring the spontaneous alternation behaviour of the mice in the Y-maze (Maurice et al., 1994, 1996, 1998). The maze was made of grey polyvinylchloride. Each arm was 40 cm long, 13 cm high, 3 cm wide at the bottom, 10 cm wide at the top and converged at an equal angle. Each mouse was placed at the end of one arm and allowed to move freely through the maze during an 8 min session. The series of arm entries, including possible returns into the same arm, were checked using an Apple IIe computer. An alternation was defined as entries into all three arms on consecutive occasions. The number of maximum alternations was, therefore, the total number of arm entries minus two and the percentage of alternation was calculated as (actual alternations/maximum alternations) × 100.
Step-through type passive avoidance response
The contextual long-term memory of the animals was assessed using the step-through passive avoidance procedure (Maurice et al., 2006; Meunier et al., 2006). The apparatus consisted of an illuminated compartment with white polyvinylchloride walls (15 × 20 × 15 cm high), a darkened compartment with black polyvinylchloride walls (15 × 20 × 15 cm high) and a grid floor. A guillotine door separated each compartment. A 60 W lamp positioned 40 cm above the apparatus lit the white compartment during the experimental period. Scrambled foot shocks (0.3 mA for 3 s) were delivered to the grid floor using a shock generator scrambler (Lafayette Instruments, Lafayette, MA, USA). The guillotine door was initially closed during the training session. Each mouse was placed into the white compartment. After 5 s, the door was raised. When the mouse entered the darkened compartment and placed all its paws on the grid floor, the door was gently closed and the scrambled foot shock was delivered for 3 s. The step-through latency, that is, the latency spent to enter the dark compartment, and the number of vocalizations was recorded. The number of vocalizations did not differ between the groups, indicating that shock sensitivity was unaffected by the i.c.v. or i.p. treatments (data not shown). The retention test was carried out 24 h after training. Each mouse was placed again into the white compartment. After 5 s, the door was raised. The step-through latency was recorded up to 300 s. Animals entered the darkened compartment or were gently pushed into it and the escape latency, that is, the time spent to return into the white compartment, was also measured up to 300 s. The two parameters were measured although they do not rely on similar mechanisms. The step-through latency involves contextual reinforced stimuli and is a direct measure of passive avoidance behaviour. The escape latency relies on supplementary sensory information, the contact with the grid floor that per se activates specific retrieval pathways, but includes conflicting information: the absence of an electric shock in this compartment during the retention session. This parameter is more reliably measured in active avoidance paradigms and may, in our case, lead to less-sensitive differences between the groups.
Lipid peroxidation measures (modified ferrous oxidation-xylenol orange (FOX) assay)
The quantification of lipid peroxidation in tissue extracts is based on Fe(III)xylenol orange complex formation according to Hermes-Lima et al. (1995). Mice were killed by decapitation and brains were rapidly removed, weighed and kept in liquid nitrogen until assayed. After being thawed, homogenates were homogenized in cold methanol (1/5 w/v), centrifuged at 1000 g for 5 min and the supernatant was placed in an eppendorf tube. The reaction volume was determined in preliminary experiments. Increasing homogenate volumes (2–100 μl) prepared from control Swiss animals were sequentially added to FeSO4 1 mM, H2SO4 0.25 M, xylenol orange 1 mM and incubated overnight in a dark chamber at room temperature. Absorbance was measured at 580 nm, and the reaction volume was determined for an absorbance value of 0.7. Then, the reaction volume of each homogenate was added to FeSO4 1 mM, H2SO4 0.25 M, xylenol orange 1 mM and incubated for 30 min at room temperature. After the absorbance had been read at 580 nm (A5801), 5 μl of cumene hydroperoxide (CHP) 1 mM was added to the sample and it was incubated for 30 min at room temperature, to determine the maximal oxidation level. The absorbance was measured at 580 nm (A5802). The level of lipid peroxidation was determined as CHP equivalents according to: CHPE=A5801/A5802 × (CHP (nmol)) and expressed as CHP equivalents per wet weight of tissue.
Drugs
Donepezil hydrochloride was obtained from Eisai Co. Ltd (Tokyo, Japan). 2-(4-Morpholino)ethyl 1-phenylcyclohexane-1-carboxylate (PRE-084) was provided by Dr Tsung-Ping Su (IRP, NIDA, NIH, Baltimore, MD, USA) and N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine (BD1047) by Dr Wayne D Bowen (Brown University, Providence, RI, USA). Rivastigmine tartrate was from Novartis (Basel, Switzerland). 9-Amino-1,2,3,4-tetrahydroacridine hydrochloride (tacrine) and galantamine hydrobromide and other chemical reagents, including xylenol orange and cumene hydroperoxide, were from Sigma-Aldrich (St-Quentin-Fallavier, France). Doses refer to the salt form. The range of doses of the drug used were selected on the basis of those used in previous studies examining the anti-amnesic or neuroprotective effects of donepezil, PRE-084 or AChE inhibitors in pharmacological, hypoxic or Aβ25–35 models of amnesia (Maurice et al., 1998, 2006; Meunier et al., 2006). Compounds were injected intraperitoneally (i.p.) in a volume of 100 μl per 20 g of body weight. For antagonism studies, the σ1 receptor antagonist was administered before each drug and control animals received only one injection of vehicle (saline solution), as in numerous previous studies no differences in behavioural responses were observed after one or two injections of saline (i.p., data not shown). The amyloid β25–35 peptide (Aβ25–35, SC489C) and scrambled Aβ25–35 peptide (SC492) were from NeoMPS (Strasbourg, France). They were dissolved in sterile bidistilled water at a concentration of 3 mg ml−1 and stored at −20°C until use. Before being injected, peptides were aggregated by incubation at 3 mg ml−1 in sterile bidistilled water at 37°C for 4 days. They were administered intracerebroventricularly (i.c.v.), according to the method of Haley and McCormick (1957), in a final volume of 3 μl per mouse, as previously described (Maurice et al., 1996, 1998).
Statistical analyses
Y-maze test data and lipid peroxidation measures were expressed as mean value±s.e.m. and analysed using Dunnett's or Newman–Keuls' multiple comparisons test after a one-way analysis of variance (ANOVA, F-values). Passive avoidance latencies did not show a normal distribution, as a cutoff time was set. They were thus expressed as median value and interquartile range and analysed using the Kruskal–Wallis non-parametric ANOVA (H-values), group comparisons being made with Dunn's non-parametric multiple comparisons test. The level of statistical significance was P<0.05.
Results
Amnesic effects of Aβ25–35 peptide administration in mice
Aβ25–35 peptide, administered i.c.v., provoked marked learning impairments in mice after 1 week, as shown in Figure 2. Aβ25–35 peptide, 1, 3 or 9 nmol per mouse i.c.v. dose-dependently, diminished the spontaneous alternation performance in the Y-maze (Figure 2a), without affecting significantly the locomotor response (Figure 2b). In parallel, passive avoidance deficits were observed, both the step-through latency (assessed using the entry into the dark compartment) and the escape latency (measured by the return in to the white compartment) showed dose-dependent diminutions as compared to scrambled Aβ25–35-treated mice (Figure 2c and d).
Figure 2.
Dose–response effect of Aβ25–35 peptide in mice. The Aβ25–35 peptide (Aβ, 1, 3 or 9 nmol) or the scrambled Aβ25–35 peptide (Sc.Aβ, 9 nmol) was administered i.c.v. The spontaneous alternation behaviour was examined on day 7: (a) spontaneous alternation percentage; (b) total number of arm entries. The step-through passive avoidance training was carried out on day 8 and retention examined on day 9: (c) step-through latency; (d) escape latency. The number of animals per group was n=10. One-way ANOVA: F(3,36)=7.24, P<0.001 in (a), F(3,36)=2.06, P>0.05 in (b). Kruskal–Wallis ANOVA: H=9.96, P<0.05 in (c), H=12.91,P<0.01 in (d). *P<0.05, **P<0.01 vs Sc.Aβ-treated group, Dunnett's test in (a), Dunn's test in (c, d).
Anti-amnesic effects of donepezil and other drugs against Aβ25–35 peptide-induced amnesia in mice
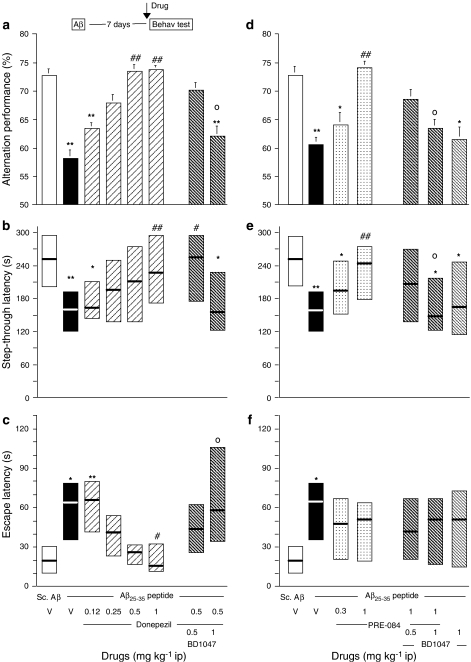
Donepezil, PRE-084 and the ChE inhibitors tacrine, rivastigmine and galantamine were injected (i.p.) 20 min before the Y-maze test session or 20 min before the passive avoidance training session, that is, 7 or 8 days, respectively, after Aβ25–35 administration in mice. Donepezil was tested in the dose range 0.12–1 mg kg−1. As shown in Figure 3a, it significantly reversed the alternation deficits at 0.5 and 1 mg kg−1. The effect of donepezil at 0.5 mg kg−1 was blocked by pretreatment with BD1047 (Figure 3a). Donepezil also dose-dependently attenuated the Aβ25–35 peptide-induced passive avoidance deficits, both in terms of step-through latency (Figure 3b) and escape latency (Figure 3c). The effect of donepezil, 0.5 mg kg−1, was attenuated, for the step-through latency parameter, or significantly antagonized, for the escape latency parameter, by pretreatment with the highest dose of BD1047 (Figure 3b and c).
Figure 3.
Beneficial effects of donepezil (a–c) and PRE-084 (d–f) against Aβ25–35 peptide-induced amnesia in mice. Mice were administered i.c.v. with Aβ25–35 peptide (9 nmol) or scrambled Aβ25–35 peptide (Sc.Aβ, 9 nmol). On day 7, animals were examined for spontaneous alternation performances (a and d). On day 8, animals were trained for passive avoidance task and retention was examined on day 9, in terms of step-through latency (b and e) and escape latency (c and f). Vehicle solution (saline, V), donepezil (0.12–1 mg kg−1), PRE-084 (0.3–1 mg kg−1) and/or BD1047 (0.5–1 mg kg−1) were administered i.p. 20 min before the test (see insert). n=10 per group. F(7,79)=4.26, P<0.001 in (a); H=16.01, P<0.05 in (b); H=32.34, P<0.0001 in (c); F(6,63)=4.15, P<0.01 in (d); H=15.87, P<0.05 in (e); H=13.08, P<0.05 in (f). *P<0.05, **P<0.01 vs the V-treated Sc.Aβ-administered group; #P<0.05, ##P<0.01 vs the V-treated Aβ23–35-administered group; oP<0.05 vs the donepezil (0.5 mg kg−1)- or PRE-084 (1 mg kg−1)-treated Aβ23–35-administered group; Dunnett's test in (a, d), Dunn's test in (b, c, e, f).
PRE-084, the reference σ1 receptor agonist, was administered at 0.3 and 1 mg kg−1. At the highest dose tested, the compound reversed the Aβ25–35-induced alternation deficits (Figure 3d). This effect of PRE-084 was blocked by the σ1 receptor antagonist BD1047, which had no effect by itself (Figure 3d). In the passive avoidance test, the highest dose of PRE-084 significantly reversed the Aβ25–35-induced decrease in step-through latency (Figure 3e) and nonsignificantly attenuated the Aβ25–35-induced increase in escape latency (Figure 3f). Pretreatment with BD1047 significantly blocked the effects of PRE-084 on step-through latency (Figure 3e), but not its effects on escape latency (Figure 3f).
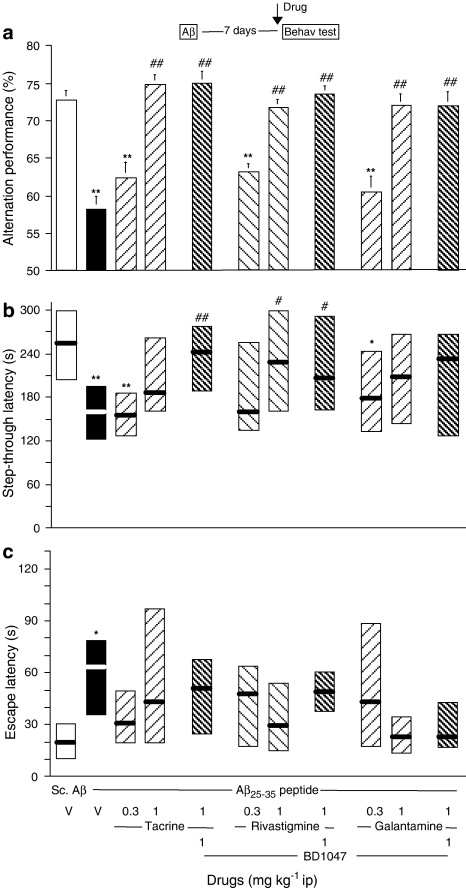
Tacrine, rivastigmine and galantamine were also tested. All three ChE inhibitors highly significantly reversed the Aβ25–35-induced alternation deficits, at the highest doses tested, 1 mg kg−1. Interestingly, the effects were unaffected by BD1047 pretreatment, even at the highest dose of this antagonist (Figure 4a). The passive avoidance procedure led to similar results, but with lower levels of significance. At 1 mg kg−1, the three compounds attenuated the Aβ25–35-induced decrease in step-through latency, but only the rivastigmine effect reached significance (Figure 4b). The effects were not affected by BD1047. Differences in terms of escape latency were very mild. As only the vehicle-treated Aβ25–35 group showed a significant increase of latency as compared with the vehicle-treated scrambled Aβ25–35 control group, it is probable that the drug treatments tended to attenuate the Aβ25–35-induced deficits (Figure 4c).
Figure 4.
Beneficial effects of tacrine, rivastigmine and galantamine against Aβ25–35 peptide-induced amnesia in mice. Mice were administered i.c.v. with Aβ25–35 peptide (9 nmol) or scrambled Aβ25–35 peptide (Sc.Aβ, 9 nmol). After 7 days, animals were examined for spontaneous alternation performances (a). On day 8, animals were trained for passive avoidance task and retention was examined on day 9, in terms of step-through latency (b) and escape latency (c). Vehicle solution (saline, V), tacrine (0.3–1 mg kg−1), rivastigmine (0.3–1 mg kg−1), galantamine (0.3–1 mg kg−1) and/or BD1047 (1 mg kg−1) were administered i.p. 20 min before the test. n=10 per group. F(10,99)=4.38, P<0.0001 in (a); H=27.09, P<0.05 in (b); H=25.74, P<0.05 in (c). *P<0.05, **P<0.01 vs the V-treated Sc.Aβ-administered group; #P<0.05, ##P<0.01 vs the V-treated Aβ25–35-administered group; Dunnett's test in (a), Dunn's test in (b, c).
Neuroprotective effects of donepezil and other drugs against Aβ25–35 peptide-induced toxicity after pre-i.c.v. administration
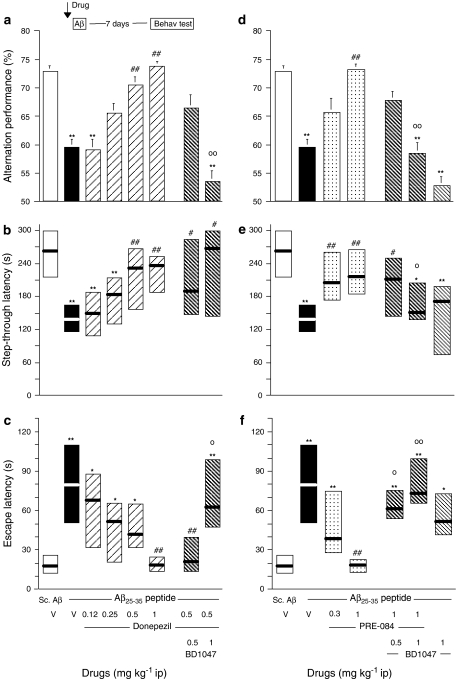
Compounds were injected i.p. 20 min before the peptide and the behavioural observations were initiated 7 days (Y-maze test session) or 8 days (passive avoidance training) later. The lipid-peroxidized products were measured in mice that did not experience the behavioural tests, 7 days after Aβ25–35 administration. Donepezil, when tested at 0.12–1 mg kg−1 i.p., significantly reversed the alternation deficits at 0.5 and 1 mg kg−1 (Figure 5a). The effect of donepezil at 0.5 mg kg−1 was completely blocked by pretreatment with BD1047 (Figure 5a). Donepezil also dose-dependently attenuated the Aβ25–35 peptide-induced passive avoidance deficits, both in terms of step-through latency (Figure 5b) and escape latency (Figure 5c). The effect of donepezil, 0.5 mg kg−1, was unaffected, for the step-through latency parameter, but significantly blocked, for the escape latency parameter, by pretreatment with the highest dose of BD1047 (Figure 5b and c).
Figure 5.
Pre i.c.v. neuroprotective effects of donepezil (a–c) and PRE-084 (d–f) in Aβ25–35 peptide-injected mice. Mice were administered, i.p., either vehicle solution (saline, V), donepezil (0.12–1 mg kg−1), PRE-084 (0.3–3 mg kg−1) and/or BD1047 (0.5–3 mg kg−1) 20 min before being administered i.c.v. with Aβ25–35-peptide (9 nmol) (see insert). Control animals received scrambled Aβ25–35 peptide (Sc.Aβ, 9 nmol). After 7 days, animals were examined for spontaneous alternation performances (a and d). On day 8 after peptide injection, animals were trained for passive avoidance task and retention was examined on day 9, in terms of step-through latency (b and e) and escape latency (c and f). n=10–12 per group. F(7,77)=5.93, P<0.0001 in (a); H=27.60, P<0.001 in (b); H=31.13, P<0.0001 in (c); F(6,67)=6.28, P<0.0001 in (d); H=22.89, P<0.001 in (e); H=31.31, P<0.0001 in (f). *P<0.05, **P<0.01 vs the V-treated Sc.Aβ-administered group; #P<0.05, ##P<0.01 vs the V-treated Aβ25–35-administered group; oP<0.05, ooP<0.01 vs the donepezil (0.5 mg kg−1)- or PRE-084 (1 mg kg−1)-treated Aβ25–35-administered group; Dunnett's test in (a, d), Dunn's test in (b, c, e, f).
PRE-084 was again administered at 0.3 and 1 mg kg−1. At the highest dose tested, it prevented the Aβ25–35-induced alternation deficits (Figure 5d). This effect of PRE-084 was dose-dependently blocked by BD1047, the compound being devoid of effect by itself (Figure 5d). In the passive avoidance test, PRE-084 significantly reversed the Aβ25–35-induced decrease in step-through latency (Figure 5e) and increase in escape latency (Figure 5f). Pretreatment with BD1047 significantly blocked the PRE-084 effects on both parameters (Figure 5e and f).
Tacrine, rivastigmine and galantamine, when injected before the Aβ25–35 peptide, failed to affect the resulting alternation deficits (Figure 6a). In the passive avoidance procedure, analysis of the step-through latency led to similar results (Figure 6b). Rivastigmine, but not tacrine or galantamine, allowed a significant amelioration of the escape latency, at the highest dose tested (Figure 6c). Nevertheless, the ChE inhibitors were overall poorly active.
Figure 6.
Pre-i.c.v. neuroprotective effects of tacrine, rivastigmine and galantamine in Aβ25–35 peptide-injected mice. Mice were administered, i.p., either vehicle solution (saline, V), tacrine (0.3–1 mg kg−1), rivastigmine (0.3–1 mg kg−1) or galantamine (0.3–1 mg kg−1) 20 min before being administered i.c.v. with Aβ25–35 peptide (9 nmol) (see insert). Control animals received scrambled Aβ25–35 peptide (Sc.Aβ, 9 nmol). After 7 days, animals were examined for spontaneous alternation performances (a). On day 8 after peptide injection, animals were trained for passive avoidance task and retention was examined on day 9, in terms of step-through latency (b) and escape latency (c). n=10–12 per group. F(7,76)=2.94, P<0.05 in (a); H=16.63, P<0.05 in (b); H=28.71, P<0.001 in (c). *P<0.05, **P<0.01 vs the V-treated Sc.Aβ-administered group; ##P<0.01 vs the V-treated Aβ25–35-administered group; Dunnett's test in (a), Dunn's test in (b, c).
The neuroprotective effects of the drugs were also tested on the oxidative stress response induced by Aβ25–35 peptide (Figure 7). Aβ25–35 peptide augmented the levels of lipid-peroxidized products in the mouse hippocampus 7 days after injection (+83%). Pre-administration of donepezil (0.5 mg kg−1, i.c.v.) or PRE-084 (1 mg kg−1, i.c.v.), at their behaviourally active doses, resulted in a complete blockade of the lipid peroxidation augmentation (Figure 7a). Pre-administration of BD1047 significantly blocked the donepezil and PRE-084 effects, the σ1 receptor antagonist being without effect by itself (Figure 7a). Tacrine, rivastigmine or galantamine, tested at 1 mg kg−1, failed to show any effect (Figure 7b).
Figure 7.
Pre-i.c.v. neuroprotective effects of the compounds, assessed using measures of the lipid peroxidation levels in the hippocampus of Aβ25–35 peptide-injected mice. Mice were administered, i.p., either vehicle solution (saline, V), donepezil (0.5 mg kg−1), PRE-084 (1 mg kg−1) and/or BD1047 (1 mg kg−1) (a); or tacrine (1 mg kg−1), rivastigmine (1 mg kg−1), galantamine (1 mg kg−1) and/or BD1047 (1 mg kg−1) (b) 20 min before being administered i.c.v. with Aβ25–35 peptide (9 nmol) (see insert). Lipid peroxidation levels were measured on day 7. n=6–8 per group. F(6,39)=16.21, P<0.0001 in (a); F(4,29)=7.54, P<0.001 in (b). **P<0.01 vs the V-treated Sc.Aβ-administered group; ##P<0.01 vs the V-treated Aβ25–35-administered group, oo P<0.01 vs the donepezil (0.5 mg kg−1)- or PRE-084 (1 mg kg−1)-treated Aβ25–35-administered group; Newman–Keuls' test.
Neuroprotective effects of donepezil and other drugs against Aβ25–35 peptide-induced toxicity post-i.c.v. administration
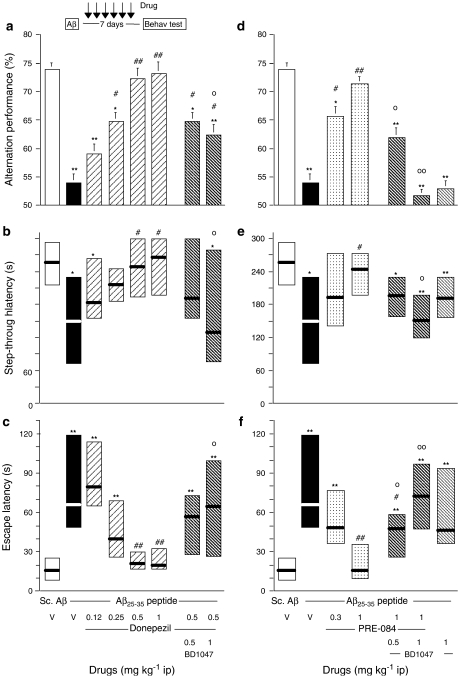
We finally examined the neuroprotective effects of the compounds injected 24 h after the peptide and once a day for 6 days (before the Y-maze test session) or 7 days (before the passive avoidance training). The lipid-peroxidized products were measured in mice that did not experience the behavioural tests, 7 days after Aβ25–35 administration. Donepezil, when tested at 0.12–1 mg kg−1 i.p., significantly reversed the alternation deficits at 0.5 and 1 mg kg−1 (Figure 8a). The effect of donepezil at 0.5 mg kg−1 was partially, but significantly attenuated by pretreatment with BD1047 (Figure 8a). Donepezil also dose-dependently attenuated the Aβ25–35 peptide-induced decrease in step-through latency (Figure 8b) and increase in escape latency (Figure 8c) in the passive avoidance procedure. The effects of donepezil, 0.5 mg kg−1, on both parameters, were blocked by pretreatment with the highest dose of BD1047 (Figure 8b and c).
Figure 8.
Post i.c.v. neuroprotective effects of donepezil (a–c) and PRE-084 (d–f) in Aβ25–35 peptide-injected mice. Mice were administered, i.p., either vehicle solution (saline, V), donepezil (0.12–1 mg kg−1), PRE-084 (0.3–1 mg kg−1) and/or BD1047 (0.5–1 mg kg−1) 24 h after the peptide injection and once a day for 6 days (see insert). On day 7, animals were examined for spontaneous alternation performances (a, d). On day 8, animals were trained for passive avoidance task and retention was examined on day 9, in terms of step-through latency (b, e) and escape latency (c, f). n=10–12 per group. F(7,83)=5.61, P<0.0001 in (a); H=15.63, P<0.05 in (b); H=38.79, P<0.0001 in (c); F(6,71)=11.20, P<0.0001 in (d); H=16.74, P<0.05 in (e); H=33.59, P<0.0001 in (f). *P<0.05, **P<0.01 vs the V-treated Sc.Aβ-administered group; #P<0.05, ##P<0.01 vs the V-treated Aβ25–35-administered group; oP<0.05, ooP<0.01 vs the donepezil (0.5 mg kg−1)- or PRE-084 (1 mg kg−1)-treated Aβ25–35-administered group; Dunnett's test in (a, d), Dunn's test in (b, c, e, f).
PRE-084 dose-dependently prevented the Aβ25–35-induced alternation deficits (Figure 8d) and passive avoidance deficits (Figure 8e and f). These effects of PRE-084 were dose-dependently blocked by BD1047, the compound being devoid of effect by itself (Figure 8d–f).
Tacrine, rivastigmine and galantamine were also effective in blocking the Aβ25–35-induced toxicity when administered post i.c.v. Tacrine and galantamine, but not rivastigmine, attenuated the Aβ25–35-induced spontaneous alternation deficits (Figure 9a). The effects were unaffected by BD1047 (Figure 9a). Tacrine, rivastigmine and galantamine ameliorated the passive avoidance deficits induced by Aβ25–35, both in terms of step-through latency (Figure 9b) or escape latency (Figure 9c). Pretreatment with BD1047 failed to affect the effects of these drugs significantly. Interestingly, with this administration procedure, galantamine was more potent at 0.3 mg kg−1 than at 1 mg kg−1 (Figure 9a–c).
Figure 9.
Post-i.c.v. neuroprotective effects of tacrine, rivastigmine and galantamine in Aβ25–35 peptide-injected mice. Mice were administered, i.p., either vehicle solution (saline, V), tacrine (0.3–1 mg kg−1), rivastigmine (0.3–1 mg kg−1), galantamine (0.3–1 mg kg−1) and/or BD1047 (1 mg kg−1) 24 h after the peptide injection and once a day for 6 days (see insert). On day 7, animals were examined for spontaneous alternation performances (a). On day 8, animals were trained for passive avoidance task and retention was examined on day 9, in terms of step-through latency (b) and escape latency (c). n=10–12 per group. F(10,103)=3.82, P<0.001 in (a); H=19.23, P<0.05 in (b); H=41.65, P<0.0001 in (c). *P<0.05, **P<0.01 vs the V-treated Sc.Aβ-administered group; #P<0.05, ##P<0.01 vs the V-treated Aβ25–35-administered group; Dunnett's test in (a), Dunn's test in (b, c).
The neuroprotective effects of the drugs post i.c.v. administration were also tested on the oxidative stress response induced by Aβ25–35 peptide injection (Figure 10). Donepezil (0.5 mg kg−1) or PRE-084 (1 mg kg−1), at their behaviourally active doses, significantly attenuated the Aβ25–35 peptide-induced lipid peroxidation (−80 and −70%, respectively, Figure 10a). Preadministration of BD1047 decreased this effect of donepezil (40% reduction), but not significantly, whereas the effect of PRE-084 was completely and significantly blocked. The σ1 receptor antagonist was devoid of effect by itself (Figure 10a). Tacrine, rivastigmine and galatamine, tested at 1 mg kg−1, also showed some efficacy in preventing the increase in lipid-peroxidized products formation and this was significant for rivastigmine and galantamine (Figure 10b). The BD1047 pretreatment did not affect these drug effects (Figure 10b).
Figure 10.
Post-i.c.v. neuroprotective effects of the compounds, assessed using measures of the lipid peroxidation levels in the hippocampus of Aβ25–35 peptide-injected mice. Mice were administered, i.p., either vehicle solution (saline, V), donepezil (0.5 mg kg−1), PRE-084 (1 mg kg−1) and/or BD1047 (1 mg kg−1) (a); or tacrine (1 mg kg−1), rivastigmine (1 mg kg−1), galantamine (1 mg kg−1) and/or BD1047 (1 mg kg−1) (b) 24 h after the peptide injection and once a day for 6 days (see insert). Lipid peroxidation levels were measured on day 7. n=6–8 per group. F(6,39)=6.38, P<0.0001 in (a); F(7,44)=3.77, P<0.01 in (b). *P<0.05, **P<0.01 vs the V-treated Sc.Aβ-administered group; #P<0.05, ##P<0.01 vs the V-treated Aβ25–35-administered group, oP<0.05; Newman–Keuls' test.
Discussion
The Aβ protein, the major component of neuritic plaques found in AD, has been implicated as a potential contributor to the disease's progressive neuropathology. After in vitro exposure to aggregates of synthetic Aβ peptide, the neurites of rat-cultured hippocampal neurons adopt a dystrophic appearance. The morphological changes in the neurites include beading, fragmentation, terminal swelling and tortuous growth patterns. The degenerative changes are similar to those observed in neurites associated with neuritic plaques, suggesting that Aβ may induce the neuritic abnormalities of AD neuropathology (Pike et al., 1991). The truncated Aβ25–35 fragment includes extracellular and transmembrane residues that have been reported to represent an active region of Aβ (Yankner et al., 1990). Structure–activity studies revealed that peptides containing the highly hydrophobic (29–35) region formed stable aggregations (Pike et al., 1993). Numerous in vitro studies have provided evidence that Aβ25–35 induces neuronal death by necrosis or apoptosis (Behl et al., 1994; Ivins et al., 1999), resulting from exposure to the peptide. Moreover, a correlation between the ability of Aβ peptide fragments to self-aggregate and their neurotoxicity was observed in long-term neuronal cultures, consistent with the hypothesis that Aβ protein aggregation contributes to neurodegeneration in AD (Pike et al., 1993).
Two nontransgenic rodent models of AD have been studied in the past 10 years to analyse the molecular, morphological and behavioural consequences of amyloid toxicity in vivo, namely the infusion of either Aβ1–40/42 protein or Aβ25–35 peptide. A rapid review of the literature revealed the parallels between these two strategies that relied on the use of the endogenous amyloid protein or a synthetic neurotoxic peptide. After 14 days of an i.c.v. infusion of Aβ1–40 protein, immunohistochemical accumulation of the protein was observed throughout the hippocampus and cerebral cortex (Nitta et al., 1994). The immunolabelling of Aβ25–35, after i.c.v. injection, has not yet been reported. However, Congo red-stained deposits have been observed throughout the hippocampal formation and cortex (Maurice et al., 1996), with a similar morphological aspect as that observed for the Aβ1–40 protein (Giovannelli et al., 1998). Indeed, Kowall et al. (1992) initially reported that both Aβ1–40 and Aβ25–35 injected into the rat cortex produced localized necrosis at the injection site surrounded by a zone of neuronal loss and gliosis. Morphological damages in the CA1–2 and dendate gyrus areas of the hippocampus, together with increased GFAP immunoreactivity, were observed 2 weeks after cessation of an i.c.v. infusion of Aβ1–40 (Nitta et al., 1997). Aβ1–42, administered i.c.v., increased the immunoreactivities of glial fibrillary acidic protein (GFAP), the astrocyte marker, and interleukin-1β in the hippocampus (Cho et al., 2005). Aβ25–35 also causes reactive gliosis in the ipsilateral hemisphere, as demonstrated by upregulation of GFAP expression and the presence of hypertrophic astrocytes in the hippocampus (Stepanichev et al., 2003). After caspase-3 activity had been induced in the hippocampus and cortex of rats (Stepanichev et al., 2003), an injection of Aβ25–35 produced a moderate but significant reduction in the number of neurons in the CA1 or CA3 hippocampal areas (Stepanichev et al., 2003, 2005, 2006; Mamiya et al., 2004; unpublished results).
Oxidative stress contributes to the Aβ1–42-induced toxicity in vivo, as shown by induction of cytosolic Cu,Zn-superoxide dismutase (SOD) and mitochondrial Mn-SOD in the hippocampus and cortex. Production of malondialdehyde (lipid peroxidation) and protein carbonyl (protein oxidation) remains elevated 10 days after Aβ1–42 injection (Jhoo et al., 2004). Reduction of SOD immunoreactivity was also clearly evidenced after Aβ1–40 fusion (Kim et al., 2003). Chronic Aβ1–40 infusion caused a robust peroxynitrite formation and subsequent tyrosine nitration of proteins, particularly synaptophysin, in the hippocampus (Tran et al., 2003). Similarly, Aβ25–35 induces significant oxidative stress, measured within 1 week after injection, as an increase in lipid peroxidation and superoxide generation (Mamiya et al., 2004; Stepanichev et al., 2004; this study).
In both models, the amyloid toxicity directly affects neuronal physiology. Cholinergic and glutamatergic systems appear to be the most sensitive ones. The impact of Aβ peptides on cholinergic systems was studied mainly by biochemical techniques. Choline acetyltransferase activity, nicotine-induced acetylcholine release and nicotine- and high K+-induced dopamine release were significantly decreased in the frontal cortex and hippocampus of Aβ1–40-infused rats (Nitta et al., 1994; Itoh et al., 1996). The effects of Aβ25–35 were examined after its chronic infusion and it was shown to decrease nicotine-evoked acetylcholine release from the frontal cortex/hippocampus of rats and reduce protein kinase C (PKC) activation, measured as a decrease in [3H]phorbol dibutyrate binding (Olariu et al., 2001). The impact of Aβ peptides on glutamatergic systems was examined by use of an electrophysiological approach. The amplitude of field excitatory postsynaptic potentials recorded in the CA1 region of awake rats was reduced 24 h after the injection of Aβ1–40 and this effect was prevented by treatment with N-methyl-D-aspartate (NMDA) receptor antagonists, suggesting that Aβ1–40 produced a delayed reduction in the function of glutamatergic synapses, probably as a result of an initial overactivation of the NMDA receptor-mediated component of transmission (Cullen et al., 1996). Itoh et al. (1999) confirmed that long-term potentiation induced by tetanic stimulations in CA1 pyramidal cells, which was readily observed in the vehicle control rats, was also impaired in the Aβ1–40-infused rats. Similarly, i.c.v. administration of aggregated Aβ25–35 was followed 1 month later by significant changes in the dynamics of long-term potentiation in the hippocampus in vivo, expressed as powerful and stable increases in the amplitude of evoked potentials (Trubetskaya et al., 2003).
These Aβ-induced toxicity and functional deficits are responsible for the delayed learning and memory deficits observed. Continuous i.c.v. infusion of Aβ1–40 induced memory impairments in the water-maze task and passive avoidance test when compared with control Aβ40–1 infused rats (Nitta et al., 1994, 1997). Single bilateral i.c.v. injection of Aβ25–35 in male Wistar rats induced, after 1month, learning impairments in the radial-arm maze, which appeared to be more marked for the working memory component than for reference memory in the water-maze test or passive avoidance test (Stepanichev et al., 2003, 2005, 2006). However, another study has clearly demonstrated reference memory impairment in the water-maze after an i.c.v. injection of Aβ25–35 (15 nmol) in Wistar rats (Delobette et al., 1997). Moreover, Aβ25–35-induced deficits in passive avoidance response, spontaneous alternation and place learning in the water-maze have repeatedly been demonstrated in mice (Maurice et al., 1996, 1998; Mamiya et al., 2004; this study). Interestingly, the Aβ25–35-induced deficits in rats were still observable 6 months after injection in a spontaneous alternation and social recognition test (Stepanichev et al., 2003).
In the present study, we therefore use the validated model of aggregated Aβ25–35 peptide injection in mice to examine the anti-amnesic and neuroprotective effects of donepezil, in comparison with other, more selective, ChE inhibitors and a reference σ1 receptor agonist. Donepezil, with a 14.6 nM affinity for the σ1 receptor and an IC50 of 21.5 nM for inhibition of acetylcholinesterase activity, has been shown to be equipotent for the two targets (Kato et al., 1999). Other cholinesterase inhibitors are more selective cholinomimetics and have only very low affinity for the σ1 receptor. For instance, tacrine shows an affinity of 6 μM for the σ1 receptor (Kato et al., 1999) as compared with an IC50 of 77 nM for the inhibition of acetylcholinesterase activity (Ogura et al., 2000). In parallel, PRE-084 is a poor muscarinic ligand, with an affinity for [3H]quinuclidinyl benzilate-binding sites of about 14 μM (Su et al., 1991) and no reported affinity for nicotinic receptors or AChE. The purpose of this study was, firstly, to identify any neuroprotective effect of donepezil on an in vivo nontransgenic model of AD; secondly, to analyse whether the interaction of the compound with the σ1 receptor is involved in its putative neuroprotective activity and thirdly, to demonstrate the neuroprotective potential of σ1 receptor agonists against Aβ toxicity in vivo.
In the first part of the study, we observed that donepezil, the selective ChE inhibitors, tacrine, rivastigmine and galantamine, and the σ1 receptor agonist PRE-084, all have potent anti-amnesic activity against the learning deficits induced by i.c.v. injection of aggregated Aβ25–35 peptide in mice. In animals treated with Aβ25–35 1 week before, the acute pre-test injections of the compounds allowed recovery of spontaneous alternation or passive avoidance deficits. These results confirm previous similar observations. Firstly, donepezil, administered at 2.5 mg kg−1 per os in rats, alleviated the deficits of delayed-matching to position paradigm in rats infused bilaterally with Aβ1–40 peptide into the hippocampus (Yamada et al., 2005). The symptomatic effect of donepezil was compared to that of memantine and demonstrated a complete recovery of the Aβ-induced deficits. Tacrine, or direct injection of nicotine, have been shown to alleviate the deficits of spontaneous alternation, passive avoidance and place learning in a water-maze induced by Aβ25–35 in mice (Maurice et al., 1996). Moreover, the anti-amnesic effect of PRE-084 has also been described previously in the Aβ25–35 mouse model of AD (Maurice et al., 1998). Notably, the observation that pretreatment with BD1047 significantly blocked the anti-amnesic effect of donepezil showed that the drug did not behave as a pure ChE inhibitor, but that an interaction with the σ1 receptor is involved in its behavioural action. We previously demonstrated that the anti-amnesic effect of donepezil against the learning deficits induced by blockade of the NMDA receptor in dizocilpine-treated mice (Maurice et al., 2006), or by hypoxia in CO gas-exposed mice (Meunier et al., 2006), is also blocked by the σ1 receptor antagonist. In all cases, BD1047 almost abolished the anti-amnesic effect of donepezil, suggesting that its effects on σ1 receptors and cholinergic systems are not purely additive. Donepezil appears to have a unique pharmacological action when compared with other selective cholinesterase inhibitors. This implies that drugs acting nonselectively as cholinomimetics and σ1 receptor agonists may present a very specific mode of action. Indeed, the physiological consequences of σ1 receptor activation are intracellular regulation of Ca2+ mobilization and activation of phospholipase C (PLC) and PKC pathways (Morin-Surun et al., 1999; Hayashi et al., 2000), which will in turn affect the signal transduction downstream to acetylcholine receptor activation and result in a complete blockade by selective σ1 receptor antagonists.
The second part of the study examined the neuroprotective efficacy of donepezil, ChE inhibitors and PRE-084 against Aβ25–35 peptide-induced toxicity. The toxicity was evaluated at two levels: a biochemical index of the oxidative stress induced in the hippocampus, by measure of the lipid peroxidation products, and the resulting learning deficits as a direct behavioural consequence. The protection against the Aβ25–35 peptide application was examined by administration of the drugs before the peptide, whereas the long-term protection against the delayed neurodegeneration induced by amyloid deposits was examined by administration of the drugs semichronically between the peptide injection and behavioural or biochemical measures. Both types of measure led to concordant results indicating that donepezil and PRE-084 are potent at exerting protection when administered pre-i.c.v., in a BD1047-sensitive manner, whereas other ChE inhibitors were without effect. When the drugs were injected semichronically, both σ1 and cholinomimetics showed some neuroprotective efficacy.
Donepezil has been reported to protect against Aβ1–40 or Aβ25–35 toxicity in cell culture models. In particular, this compound, as well as tacrine, protected PC12 cells from Aβ25–35 toxicity when applied 2 h before the peptide (Svensson and Nordberg, 1998). In rat septal neurons, donepezil, but not galantamine or tacrine, blocked the Aβ1–40 toxicity when added 24 h before the peptide. Selective effects of donepezil were measured in terms of LDH release, and thioflavin-T fluorescence (Kimura et al., 2005). Particularly from this last, highly relevant study, a parallel can be drawn between in vitro and in vivo studies suggesting that donepezil pre-administered before Aβ peptides induced a more effective neuroprotection than selective ChE inhibitors. Recently, the neuroprotective activities of PRE-084 and (−) MR-22, another selective σ1 receptor agonist, were also described against Aβ25–35 peptide-induced toxicity in rat cortical neurons (Marrazzo et al., 2005). Each compound was applied before Aβ25–35 and significant enhancement of cell survival and diminutions of the expression of the proapoptotic protein Bax were measured. The neuroprotective activity of selective σ1 receptor agonists, previously described in excitotoxic models in vitro and in vivo (for reviews, see Maurice and Lockhart, 1997; Maurice et al., 1999), is also effective against amyloid toxicity. Moreover, a complete reversion of Aβ25–35-induced oxidative stress and learning deficits by PRE-084 and a complete blockade of donepezil's effects by BD1047 were observed when drugs were administered pre-i.c.v. This, together with the lack of efficacy of selective ChE inhibitors, strongly suggests that the σ1 receptor is mainly involved in the neuroprotective effect of donepezil or, at least, that under these particular experimental conditions, the drug acts mainly as a σ1 receptor agonist. In other words, donepezil, through its σ1 receptor agonist property, is more efficient than other ChE inhibitors in blocking the toxic effect of newly synthetized β-amyloid peptides. Moreover, the pre-i.c.v. administration of donepezil before Aβ25–35 offers suitable conditions to examine the involvement of σ1 receptors in its effects.
When drugs were administered repeatedly after the Aβ25–35 peptide injection, the donepezil effects were partly antagonized by BD1047. Also, tacrine, rivastigmine and galantamine induced moderate, partly significant effects on both the behavioural and biochemical measures. Therefore, both cholinomimetics and σ1 receptor agonists exhibit effective neuroprotection in these experimental conditions, reflecting the neuroprotective ability against the amyloid deposits formed. Cholinomimetics, such as galantamine, have been shown to induce phosphorylation of Akt, through activation of PI3K mediated via activation of the α7 nicotinic receptor (Kihara et al., 2004). The mechanism of the σ1 receptor-mediated neuroprotection is still elusive. As the primary effect of Aβ25–35, after penetrating the neurons, is the induction of mitochondrial and ER stress and as σ1 receptors are known to be located in resting conditions on the ER and mitochondrial membranes (Hayashi et al., 2000), the σ1 receptor-mediated effect may result from an inhibition of the mitochondrial or ER stress. In particular, σ1 drugs have been shown to regulate Ca2+ mobilization and activate PLC/PKC pathways, which may help to attenuate the cellular effects of mitochondrial or ER dysfunctions (Morin-Surun et al., 1999; Hayashi et al., 2000). The σ1 receptor-mediated effects could also involve a blockade of the penetration of the peptide, putatively by long-term effects involving recomposition of intracellular compartments and membrane composition (Hayashi and Su, 2005). The precise mechanisms are currently being investigated.
Conclusions
The results from this study confirm that donepezil is able to alleviate the memory deficits induced by Aβ25–35 peptide injection in mice and show, for the first time, that the drug is able to protect against the appearance of Aβ25–35 peptide-induced toxicity, measured in terms of peroxidized lipid formation and resulting learning impairments. In particular, the compound was effective not only when it was repeatedly injected after the Aβ25–35 peptide but also when it was administered before Aβ25–35 and this, selectively through its σ1 receptor agonist action. Therefore, the development of σ1 receptor acting drugs, selective or not, may lead to original neuroprotective strategies for the treatment of β-amyloid-induced toxicity.
Acknowledgments
This work was supported by Eisai Inc. (USA). JM was recipient of a PhD grant from the Fondation pour la Recherche Médicale (Paris, France).
Abbreviations
- Aβ25–35
amyloid β25–35 peptide
- AChE
acetylcholinesterase
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- ChE
cholinesterase
- CHP
cumene hydroperoxide
- ER
endoplasmic reticulum
- GFAP
glial fibrillary acidic protein
- i.c.v.
intracerebroventricularly
- i.p.
intraperitoneally
- NMDA
N-methyl-D-aspartate
- PLC
phospholipase C
- PKC
protein kinase C
Conflict of interest
The authors state no conflict of interest.
References
- Arias E, Ales E, Gabilan NH, Cano-Abad MF, Villarroya M, Garcia AG, et al. Galantamine prevents apoptosis induced by β-amyloid and thapsigargin: involvement of nicotinic acetylcholine receptors. Neuropharmacology. 2004;46:103–114. doi: 10.1016/s0028-3908(03)00317-4. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis J, Cole GM, Schubert D. Vitamin E protects nerve cells from amyloid β protein toxicity. Biochem Biophys Res Commun. 1992;186:944–950. doi: 10.1016/0006-291x(92)90837-b. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis JB, Klier FG, Schubert D. Amyloid β peptide induces necrosis rather than apoptosis. Brain Res. 1994;645:253–264. doi: 10.1016/0006-8993(94)91659-4. [DOI] [PubMed] [Google Scholar]
- Cafe C, Torri C, Bertorelli L, Angeretti N, Lucca E, Forloni G, et al. Oxidative stress after acute and chronic application of β-amyloid fragment 25–35 in cortical cultures. Neurosci Lett. 1996;203:61–65. doi: 10.1016/0304-3940(95)12250-8. [DOI] [PubMed] [Google Scholar]
- Cho JY, Kim HS, Kim DH, Yan JJ, Suh HW, Song DK. Inhibitory effects of long-term administration of ferulic acid on astrocyte activation induced by intracerebroventricular injection of β-amyloid peptide1–42 in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:901–907. doi: 10.1016/j.pnpbp.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Anderson AJ. A potential role for apoptosis in neurodegeneration and Alzheimer's disease. Mol Neurobiol. 1995;10:19–45. doi: 10.1007/BF02740836. [DOI] [PubMed] [Google Scholar]
- Cullen WK, Wu J, Anwyl R, Rowan MJ. β-Amyloid produces a delayed NMDA receptor-dependent reduction in synaptic transmission in rat hippocampus. NeuroReport. 1996;8:87–92. doi: 10.1097/00001756-199612200-00018. [DOI] [PubMed] [Google Scholar]
- Delobette S, Privat A, Maurice T. In vitro aggregation facilities β-amyloid peptide-(25–35)-induced amnesia in the rat. Eur J Pharmacol. 1997;319:1–4. doi: 10.1016/s0014-2999(96)00922-3. [DOI] [PubMed] [Google Scholar]
- Erme M, Geula C, Ransil BJ, Mesulam MM. The acute neurotoxicity and effects upon cholinergic axons or intracerebrally injected β-amyloid in the rat brain. Neurobiol Aging. 1992;13:553–559. doi: 10.1016/0197-4580(92)90055-3. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E. Amnestic effects in mice of four synthetic peptides homologous to amyloid β protein from patients with Alzheimer disease. Proc Natl Acad Sci USA. 1991;88:3363–3366. doi: 10.1073/pnas.88.8.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forloni G, Chiesa R, Smiroldo S, Verga L, Salmona M, Tagliavini F, et al. Apoptosis mediated neurotoxicity induced by chronic application of β amyloid fragment 25–35. NeuroReport. 1993;4:523–526. doi: 10.1097/00001756-199305000-00015. [DOI] [PubMed] [Google Scholar]
- Ghribi O, Herman MM, Pramoonjago P, Spaulding NK, Savory J. GDNF regulates the Aβ-induced endoplasmic reticulum stress response in rabbit hippocampus by inhibiting the activation of gadd 153 and the JNK and ERK kinases. Neurobiol Dis. 2004;16:417–427. doi: 10.1016/j.nbd.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Giovannelli L, Scali C, Faussone-Pellegrini MS, Pepeu G, Casamenti F. Long-term changes in the aggregation state and toxic effects of β-amyloid injected into the rat brain. Neuroscience. 1998;87:349–357. doi: 10.1016/s0306-4522(98)00169-9. [DOI] [PubMed] [Google Scholar]
- Haley TJ, McCormick WJ. Pharmacological effects produced by intracerebral injections of drugs in the concious mouse, Br. Br J Pharmacol. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Maurice T, Su TP. Ca2+ signaling via sigma1-receptors: novel regulatory mechanism affecting intracellular Ca2+ concentration. J Pharmacol Exp Ther. 2000;293:788–798. [PubMed] [Google Scholar]
- Hayashi T, Su TP. σ1 Receptors (σ1 binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther. 2003;306:718–725. doi: 10.1124/jpet.103.051284. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. The potential role of sigma1 receptors in lipid transport and lipid raft reconstitution in the brain: implication for drug abuse. Life Sci. 2005;77:1612–1624. doi: 10.1016/j.lfs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M, Willmore WG, Storey KB. Quantification of lipid peroxidation in tissue extracts based on Fe(III)xylenol orange complex formation. Free Radic Biol Med. 1995;19:271–280. doi: 10.1016/0891-5849(95)00020-x. [DOI] [PubMed] [Google Scholar]
- Itoh A, Akaike T, Sokabe M, Nitta A, Iida R, Olariu A, et al. Impairments of long-term potentiation in hippocampal slices of beta-amyloid-infused rats. Eur J Pharmacol. 1999;382:167–175. doi: 10.1016/s0014-2999(99)00601-9. [DOI] [PubMed] [Google Scholar]
- Itoh A, Nitta A, Nadai M, Nishimura K, Hirose M, Hasegawa T, et al. Dysfunction of cholinergic and dopaminergic neuronal systems in β-amyloid protein-infused rats. J Neurochem. 1996;66:1113–1117. doi: 10.1046/j.1471-4159.1996.66031113.x. [DOI] [PubMed] [Google Scholar]
- Ivins KJ, Thornton PL, Rohn TT, Cotman CW. Neuronal apoptosis induced by beta-amyloid is mediated by caspase-8. Neurobiol Dis. 1999;6:440–449. doi: 10.1006/nbdi.1999.0268. [DOI] [PubMed] [Google Scholar]
- Jhoo JH, Kim HC, Nabeshima T, Yamada K, Shin EJ, Jhoo WK, et al. β-Amyloid1–42-induced learning and memory deficits in mice: involvement of oxidative burdens in the hippocampus and cerebral cortex. Behav Brain Res. 2004;155:185–196. doi: 10.1016/j.bbr.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Kato K, Hayako H, Ishihara Y, Marui S, Iwane M, Miyamoto M. TAK-147, an acetylcholinesterase inhibitor, increases choline acetyltransferase activity in cultured rat septal cholinergic neurons. Neurosci Lett. 1999;260:5–8. doi: 10.1016/s0304-3940(98)00943-4. [DOI] [PubMed] [Google Scholar]
- Kihara T, Sawada H, Nakamizo T, Kanki R, Yamashita H, Maelicke A, et al. Galantamine modulates nicotinic receptor and blocks Aβ-enhanced glutamate toxicity. Biochem Biophys Res Commun. 2004;325:976–982. doi: 10.1016/j.bbrc.2004.10.132. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Sawada H, Kimura J, Kume T, Kochiyama H, et al. Nicotinic receptor stimulation protects neurons against β-amyloid toxicity. Ann Neurol. 1997;42:159–163. doi: 10.1002/ana.410420205. [DOI] [PubMed] [Google Scholar]
- Kim HC, Yamada K, Nitta A, Olariu A, Tran MH, Mizuno M, et al. Immunocytochemical evidence that amyloid β1–42 impairs endogenous antioxidant systems in vivo. Neuroscience. 2003;119:399–419. doi: 10.1016/s0306-4522(02)00993-4. [DOI] [PubMed] [Google Scholar]
- Kimura M, Akasofu S, Ogura H, Sawada K. Protective effect of donepezil against Aβ1–40 neurotoxicity in rat septal neurons. Brain Res. 2005;1047:72–84. doi: 10.1016/j.brainres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Kowall NW, Beal MF, Busciglio J, Duffy LK, Yanker BA. An in vivo model for the neurodegenerative effects of β-amyloid and protection by substance P. Proc Natl Acad Sci USA. 1991;88:7247–7251. doi: 10.1073/pnas.88.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall NW, McKee AC, Yankner BA, Beal MF. In vivo neurotoxicity of β-amyloid β1–40 and the β25–35 fragment. Neurobiol Aging. 1992;13:537–542. doi: 10.1016/0197-4580(92)90053-z. [DOI] [PubMed] [Google Scholar]
- Lee AS. Mammalian stress response: induction of the glucose regulated protein family. Curr Opin Cell Biol. 1992;4:267–273. doi: 10.1016/0955-0674(92)90042-b. [DOI] [PubMed] [Google Scholar]
- Malouf AT. Effect of β amyloid peptides on neurons in hippocampal slice cultures. Neurobiol Aging. 1992;13:543–551. doi: 10.1016/0197-4580(92)90054-2. [DOI] [PubMed] [Google Scholar]
- Mamiya T, Asanuma T, Kise M, Ito Y, Mizukuchi A, Aoto H, et al. Effects of pre-germinated brown rice on β-amyloid protein-induced learning and memory deficits in mice. Biol Pharm Bull. 2004;27:1041–1045. doi: 10.1248/bpb.27.1041. [DOI] [PubMed] [Google Scholar]
- Marrazzo A, Caraci F, Salinaro ET, Su TP, Copani A, Ronsisvalle G. Neuroprotective effects of sigma-1 receptor agonists against β-amyloid-induced toxicity. NeuroReport. 2005;16:1223–1226. doi: 10.1097/00001756-200508010-00018. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Barger SW, Cheng B, Lieberburg I, Smith-Swintosky V, Rydel RE. β-Amyloid precursor protein metabolites and loss of neuronal Ca2+ homeostasis in Alzheimer's disease. Trends Neurosci. 1993;16:409–414. doi: 10.1016/0166-2236(93)90009-b. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Hiramatsu M, Itoh J, Kameyama T, Hasegawa T, Nabeshima T. Behavioral evidence for a modulating role of σ ligands in memory processes. I. Attenuation of dizocilpine (MK-801)-induced amnesia. Brain Res. 1994;647:44–56. doi: 10.1016/0006-8993(94)91397-8. [DOI] [PubMed] [Google Scholar]
- Maurice T, Lockhart BP. Neuroprotective and anti-amnesic potentials of sigma (σ) receptor ligands. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:69–102. doi: 10.1016/s0278-5846(96)00160-1. [DOI] [PubMed] [Google Scholar]
- Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996;706:181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- Maurice T, Meunier J, Feng B, Ieni J, Monaghan DT. Interaction with σ1 protein, but not NMDA receptor, is involved in the pharmacological activity of donepezil. J Pharmacol Exp Ther. 2006;317:606–614. doi: 10.1124/jpet.105.097394. [DOI] [PubMed] [Google Scholar]
- Maurice T, Phan VL, Urani A, Kamei H, Noda Y, Nabeshima T. Neuroactive neurosteroids as endogenous effectors for the sigma1 (σ1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81:125–155. doi: 10.1254/jjp.81.125. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP, Privat A. Sigma1 (σ1) receptor agonists and neurosteroids attenuate β25–35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience. 1998;83:413–428. doi: 10.1016/s0306-4522(97)00405-3. [DOI] [PubMed] [Google Scholar]
- Meunier J, Ieni J, Maurice T. Anti-amnesic and neuroprotective effects of donepezil against learning impairments induced in mice by exposure to carbon monoxide (CO) gas. J Pharmacol Exp Ther. 2006;317:1307–1319. doi: 10.1124/jpet.106.101527. [DOI] [PubMed] [Google Scholar]
- Morin-Surun MP, Collin T, Denavit-Saubié M, Baulieu EE, Monnet FP. Intracellular σ1 receptor modulates phospholipase C and protein kinase C activation in the brain stem. Proc Natl Acad Sci USA. 1999;96:8196–8199. doi: 10.1073/pnas.96.14.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa M, Matsuno K, Mita S. Activation of σ1 receptor subtype leads to neuroprotection in the rat primary neuronal cultures. Neurochem Int. 1998;32:337–343. doi: 10.1016/s0197-0186(97)00105-8. [DOI] [PubMed] [Google Scholar]
- Nitta A, Fukuta T, Hasegawa T, Nabeshima T. Continuous infusion of â-amyloid protein into cerebral ventricle induces learning impairment and neuronal and morphological degeneration. Jpn J Pharmacol. 1997;73:51–57. doi: 10.1254/jjp.73.51. [DOI] [PubMed] [Google Scholar]
- Nitta A, Itoh A, Hasegawa T, Nabeshima T. β-Amyloid protein-induced Alzheimer's disease animal model. Neurosci Lett. 1994;170:63–66. doi: 10.1016/0304-3940(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Ogura H, Kosasa T, Kuriya Y, Yamanishi Y. Comparison of inhibitory activities of donepezil and other cholinesterase inhibitors on acetylcholinesterase and butyrylcholinesterase in vitro. Methods Find Exp Clin Pharmacol. 2000;22:609–613. doi: 10.1358/mf.2000.22.8.701373. [DOI] [PubMed] [Google Scholar]
- Olariu A, Tran MH, Yamada K, Mizuno M, Hefco V, Nabeshima T. Memory deficits and increased emotionality induced by β-amyloid25–35 are correlated with the reduced acetylcholine release and altered phorbol dibutyrate binding in the hippocampus. J Neural Transm. 2001;108:1065–1079. doi: 10.1007/s007020170025. [DOI] [PubMed] [Google Scholar]
- Perry EK, Tomlinson BE, Blessed G, Bergman K, Gibson PH, Perry RH. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J. 1978;2:1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW. Neurodegeneration induced by β-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ, Ramezan-Arab N, Cotman CW. β-Amyloid neurotoxicity in vitro: evidence of oxidative stress but not protection by antioxidants. J Neurochem. 1997;69:1601–1611. doi: 10.1046/j.1471-4159.1997.69041601.x. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of β-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991;563:311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Walencewicz-Wasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW. Structure–activity analyses of β-amyloid peptides: contributions of the β25–35 region to aggregation and neurotoxicity. J Neurochem. 1995;64:253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994;53:438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Stepanichev MY, Moiseeva YV, Lazareva NA, Gulyaeva NV. Studies of the effects of fragment (25–35) of β-amyloid peptide on the behavior of rats in a radial maze. Neurosci Behav Physiol. 2005;35:511–518. doi: 10.1007/s11055-005-0086-1. [DOI] [PubMed] [Google Scholar]
- Stepanichev MY, Moiseeva YV, Lazareva NA, Onufriev MV, Gulyaeva NV. Single intracerebroventricular administration of amyloid-β25–35 peptide induces impairment in short-term rather than long-term memory in rats. Brain Res Bull. 2003;61:197–205. doi: 10.1016/s0361-9230(03)00118-7. [DOI] [PubMed] [Google Scholar]
- Stepanichev MY, Zdobnova IM, Zarubenko II, Lazareva NA, Gulyaeva NV. Studies of the effects of central administration of β-amyloid peptide (25–35): pathomorphological changes in the hippocampus and impairment of spatial memory. Neurosci Behav Physiol. 2006;36:101–106. doi: 10.1007/s11055-005-0167-1. [DOI] [PubMed] [Google Scholar]
- Stepanichev MY, Zdobnova IM, Zarubenko II, Moiseeva YV, Lazareva NA, Onufriev MV, et al. Amyloid-β25–35-induced memory impairments correlate with cell loss in rat hippocampus. Physiol Behav. 2004;80:647–655. doi: 10.1016/j.physbeh.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Stepanichev MYu, Lazareva NA, Onufriev MV, Mitrokhina OS, Moiseeva YuV, Gulyaeva NV. Effects of doses of fragment (25–35) of β-amyloid peptide on behavior in rats. Neurosci Behav Physiol. 1998;28:564–566. doi: 10.1007/BF02463018. [DOI] [PubMed] [Google Scholar]
- Su TP, Wu XZ, Cone EJ, Shukla K, Gund TM, Dodge AL, et al. Sigma compounds derived from phencyclidine: identification of PRE-084, a new, selective sigma ligand. J Pharmacol Exp Ther. 1991;259:543–550. [PubMed] [Google Scholar]
- Svensson AL, Nordberg A. Tacrine and donepezil attenuate the neurotoxic effect of Aβ25–35 in rat PC12 cells. NeuroReport. 1998;9:1519–1522. doi: 10.1097/00001756-199805110-00050. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su TP. Sigma-1 receptors potentiate epidermal growth factor signaling towards neuritogenesis in PC12 cells: potential relation to lipid raft reconstitution. Synapse. 2004;53:90–103. doi: 10.1002/syn.20041. [DOI] [PubMed] [Google Scholar]
- Tran MH, Yamada K, Nakajima A, Mizuno M, He J, Kamei H, et al. Tyrosine nitration of a synaptic protein synaptophysin contributes to amyloid β-peptide-induced cholinergic dysfunction. Mol Psychiatry. 2003;8:407–412. doi: 10.1038/sj.mp.4001240. [DOI] [PubMed] [Google Scholar]
- Trubetskaya VV, Stepanichev MY, Onufriev MV, Lazareva NA, Markevich VA, Gulyaeva NV. Administration of aggregated β-amyloid peptide (25–35) induces changes in long-term potentiation in the hippocampus in vivo. Neurosci Behav Physiol. 2003;33:95–98. doi: 10.1023/a:1021761310435. [DOI] [PubMed] [Google Scholar]
- Yamada K, Takayanagi M, Kamei H, Nagai T, Dohniwa M, Kobayashi K, et al. Effects of memantine and donepezil on amyloid-induced memory impairment in a delayed-matching to position task in rats. Behav Brain Res. 2005;162:191–199. doi: 10.1016/j.bbr.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid β-protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999;155:302–314. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- Zamani MR, Allen YS, Owen GP, Gray JA. Nicotine modulates the neurotoxic effect of β-amyloid protein (25–35) in hippocampal cultures. NeuroReport. 1997;8:513–517. doi: 10.1097/00001756-199701200-00027. [DOI] [PubMed] [Google Scholar]