Abstract
Background and purpose:
Diazoxide, a well-known opener of the mitochondrial ATP-sensitive potassium (mitoKATP) channel, has been demonstrated to exert cardioprotective effect against ischemic injury through the mitoKATP channel and protein kinase C (PKC). We aimed to clarify the role of PKC isoforms and the relationship between the PKC isoforms and the mitoKATP channel in diazoxide-induced cardioprotection.
Experimental approach:
In H9c2 cells and neonatal rat cardiomyocytes, PKC-ɛ activation was examined by Western blotting and kinase assay. Flavoprotein fluorescence, mitochondrial Ca2+ and mitochondrial membrane potential were measured by confocal microscopy. Cell death was determined by TUNEL assay.
Key results:
Diazoxide (100 μM) induced translocation of PKC-ɛ from the cytosolic to the mitochondrial fraction. Specific blockade of PKC-ɛ by either ɛV1-2 or dominant negative mutant PKC-ɛ (PKC-ɛ KR) abolished the anti-apoptotic effect of diazoxide. Diazoxide-induced flavoprotein oxidation was inhibited by either ɛV1-2 or PKC-ɛ KR transfection. Treatment with 5-hydroxydecanoate (5-HD) did not affect translocation and activation of PKC-ɛ induced by diazoxide. Transfection with wild type PKC-ɛ mimicked the flavoprotein-oxidizing effect of diazoxide, and this effect was completely blocked by ɛV1-2 or 5-HD. Diazoxide prevented the increase in mitochondrial Ca2+, mitochondrial depolarization and cytochrome c release induced by hypoxia and all these effects of diazoxide were blocked by ɛV1-2 or 5-HD.
Conclusions and Implications:
Diazoxide induced isoform-specific translocation of PKC-ɛ as an upstream signaling molecule for the mitoKATP channel, rendering cardiomyocytes resistant to hypoxic injury through inhibition of the mitochondrial death pathway.
Keywords: diazoxide, protein kinase c-ɛ, mitochondrial KATP channel, cardioprotection
Introduction
Diazoxide, a well-known opener of the mitochondrial ATP-sensitive potassium (mitoKATP) channel, has been demonstrated to exert protective effects against ischemic injury in hearts and cardiomyocytes (Murata et al., 2001; Zaugg et al., 2002). Regarding the signaling mechanism for its cardioprotective effect, the mitoKATP channel and protein kinase C (PKC) have been suggested as essential components (Sato et al., 1998; Liu et al., 2002; Ohnuma et al., 2002). PKCs are classified into three subfamilies, which include the conventional (α, βI, βII, γ), novel (δ, ɛ, η, θ, ι) and atypical (ζ, μ, λ) isoforms based on Ca2+ and phospholipid sensitivity (Simkhovich et al., 1998). Although the predominant isoforms in rat heart are α, β, δ, ɛ and ζ subtypes (Disatnik et al., 1994), which PKC isoforms are essential for diazoxide-induced cardioprotection during hypoxia has not been thoroughly elucidated.
Furthermore, the nature of the interrelationship between PKC activation and the mitoKATP channel opening in the mechanism of diazoxide-induced cardioprotection has not been clear. Previous studies suggest that PKC activation can modulate mitoKATP channel activation (Sato et al., 1998), based on the findings that the PKC activating phorbol ester augmented activation of the mitoKATP channel induced by diazoxide (Sato et al., 1998), and that cardioprotection by diazoxide was abolished by a blockade of the mitoKATP channel, but not by an inhibition of PKC (Ohnuma et al., 2002). On the other hand, other studies showed that PKCs were translocated and/or activated in diazoxide-treated hearts and cardiomyocytes (Wang et al., 1999; Liu et al., 2002), suggesting that diazoxide-induced opening of the mitoKATP channel is upstream of PKC activation.
The goal of the present study was to clarify the role of PKC isoforms and the relationship between the PKC isoforms and the mitoKATP channel in diazoxide-induced cardioprotection in a hypoxic injury model of cardiomyocytes, by a pharmacological approach using PKC isoform-specific inhibitors and a molecular approach using transfection of wild-type or dominant-negative mutant form of the PKC-ɛ. In this study, rat heart-derived H9c2 cell lines (H9c2 cells) were used, as they are not only convenient but also recognized as a model well-suited for the study of cardiomyocyte biology (Hescheler et al., 1991). To show the relevance of this cell line, some key experiments have been repeated in primary cultures of neonatal rat cardiomyocytes.
Materials and methods
Cell culture: primary culture of neonatal rat cardiomyocytes and cell line of H9c2
Cardiac ventricular myocytes were prepared from 1- to 2-day-old Sprague–Dawley rats as follows. The hearts were removed, and the ventricles were minced in calcium- and bicarbonate-free Hank's buffer with 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES). These tissue fragments were digested by stepwise 0.03% collagenase type II (Gibco BRL, Rockville, MD, USA) and 0.06% pancreatin (Sigma, St Louis, MO, USA). The dissociated cells were preplated for 1 h to enrich the culture with myocytes. The nonadherent myocytes were then plated at a density of 1.5 × 104 cells/mm2. Rat heart-derived H9c2 cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). Both types of cell were cultured before experimentation in Dulbecco's modified Eagle's medium containing 5.5 mM glucose supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. The cells were maintained at 37°C in the presence of 5% CO2 in a water-jacked incubator gassed with 95% O2 and 5% CO2.
Hypoxic condition
For hypoxic challenges, the cells were transferred into an anaerobic chamber (Forma Scientific, Marietta, OH, USA) maintained at 37°C with a humidified atmosphere of 5% CO2, 10% H2 and 85% N2 as previously described (Kim et al., 2003). The anaerobic environment inside the chamber was monitored with diluted methylene blue solution as an O2 indicator before and during experiments.
Soluble/particulate fractionation
Subcellular soluble/particulate fractionation of cells was performed by a method previously described (Kim et al., 2003; Moon et al., 2004). Briefly, the cells were lysed using lysis buffer (20 mM Tris-HCl (pH 7.4), 2 mM ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA), 5 mM ethylene glycol-bis-(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 5 mM dithiothreitol, 6 mM β-mercaptoethanol, 1 mM phenylmethylsulphonyl fluoride, 20 mM leupeptin, 50 μg/ml aprotinin). The particulate (membrane) and the soluble (cytosolic) fractions of the lysates were separated by centrifugation at 100 000 g for 1 h at 4°C, and the supernatant was collected for the soluble fraction. Pellets resuspended in the same volume of lysis buffer containing 1% Triton X-100 were centrifuged at 10 000 g for 10 min at 4°C, and the supernatant was collected for the particulate fraction.
Cytosolic/mitochondrial fractionation
Subcellular cytosolic/mitochondrial fractionation of cells was performed as previously described (Kim et al., 2005). Briefly, the cells were collected by centrifugation at 1000 g for 10 min at 4°C. The cell pellets were resuspended in lysis buffer (20 mM HEPES-KOH (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulphonyl fluoride, 250 mM sucrose, 10 μg/ml leupeptin and 10 μg/ml aprotinin). The cells were then homogenized in a glass homogenizer, and the nuclei and cell debris were removed by centrifugation at 1000 g for 15 min at 4°C. The supernatants were further centrifuged at 10 000 g for 15 min at 4°C, and the resulting mitochondrial pellets were resuspended in lysis buffer. The supernatants created from the 10 000 g centrifugation were centrifuged once more at 100 000 g for 1 h at 4°C, and the supernatant was collected and designated the cytosolic fraction. We verified the mitochondrial fraction by using the mitochondrial marker, cytochrome oxidase subunit IV (COX), plasma membrane fraction with the dihydropyridine receptor protein (DRP) and cytosolic fractions with actin.
Western blotting
A quantity of 40 μg protein was separated on 8–15% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), and transferred onto polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MD, USA). The membrane was blocked with 5% nonfat dry milk, and incubated with a primary antibody. The membrane was then incubated with a secondary immunoglobulin antibody conjugated with alkaline phosphate, and the band was visualized using the NBT/BCIP method (Sigma-Aldrich, St Louis, MO, USA).
In situ terminal deoxynucleotidyl transferase UTP nick end labeling (TUNEL) assay
To examine the extent of apoptotic cell death, we performed TUNEL-staining after 8 and 6 h of hypoxia in H9c2 cells and neonatal rat cardiomyocytes, respectively, as described previously (Jung et al., 2003; Kim et al., 2005). There are three groups of diazoxide treatment. (1) ‘pre': the cells were treated with diazoxide 1 h before hypoxia followed by washout during hypoxia. (2) ‘during': the cells were treated with diazoxide only during hypoxia. (3) ‘pre/during': the cells were treated with diazoxide 1 h before and during hypoxia.
Flavoprotein fluorescence
The cells were superfused with glucose-free Tyrode's solution containing the following: 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.33 mM NaH2PO4, 0.5 mM MgCl2 and 5 mM HEPES (pH 7.4). Flavoprotein fluorescence was excited at 480 nm (for 200 ms every 20 s) and emitted at 520 nm. Relative fluorescence was calibrated with signals recorded after exposure to 100 μM 2,4-dinitrophenol (DNP), which uncouples respiration from ATP synthesis, collapses the mitochondrial potential, and induces maximal oxidation. Therefore, the values of flavoprotein fluorescence were expressed as a percentage of the DNP-induced fluorescence.
Measurement of mitochondrial membrane potential
The mitochondrial membrane potential (Δψm) was assayed by measuring the accumulation of rhodamine 123 (Molecular Probes, Eugene, OR, USA), a membrane-permeable cationic fluorescent dye (Emaus et al., 1986). H9c2 cells were loaded with 1 μM rhodamine 123 in HEPES-buffered control salt solution (HCSS) containing 120 mM NaCl, 5 mM KCl, 1.6 mM MgCl2, 2.3 mM CaCl2, 15 mM glucose, 20 mM HEPES and 10 mM NaOH. The cells were incubated for 10 min at 37°C and washed three times with HCSS. The fluorescence intensity of the cell was then measured by flow cytometry (BD Biosciences, Oxford, UK).
Measurement of mitochondrial Ca2+ uptake
The mitochondrial Ca2+ level ([Ca2+]mito) was quantified after loading cells with the Ca2+ fluorophore rhod-2 (Molecular Probes, Eugene, OR, USA), a selective fluorescent indicator for mitochondrial Ca2+. Mitochondria were labeled with 100 nM Green Mito-Tracker (Molecular probes, Eugene, OR, USA). For double-labeling, H9c2 cells were incubated for 10 min in HCSS at 37°C with 100 nM Green Mito-Tracker. The 2-step cold loading/warm incubation protocol achieves exclusive loading of rhod-2 into the mitochondria (Trollinger et al., 1997). Rhod-2 fluorescence was excited at 560 nm and emitted fluorescence was collected through a 590-nm-long pass barrier filter. The fluorescence intensity of rhod-2 was quantified using image-analysis computer software (Fluoview FV300; Olympus, Japan).
Plasmid construction
Wild-type PKC-ɛ (PKC-ɛ WT) and the dominant-negative mutant form of PKC-ɛ (PKC-ɛ KR) were cloned into pHACE that contains N-terminal HA tag (Lee et al., 2003). PKC-ɛ WT constructs contained the full-length open reading frames of PKC-ɛ and PKC-ɛ KR constructs contained full-length open reading frame with a K → R point mutation in the ATP binding site.
Cell transfection
H9c2 cells were transiently transfected with either empty vector as a control (pcDNA3) or various expression vectors using Lipofectin (Invitrogen, Carlsbad, CA, USA), following the procedure recommended by the manufacturer. The cells were grown to 50∼60% confluence before transfection. The pHACE-PKC-ɛ DNA construct was mixed with the Lipofection reagent. The mixture was then added to the serum-free cell culture medium and incubated at 37°C for 8 h, which was followed by transfection to the cells.
PKC-ɛ kinase assay
Cellular proteins were extracted using PKC extraction buffer (50 mM HEPES (pH 7.5), 150 mM NaCl, 0.1% Tween 20, 1 mM EDTA, 2.5 mM EGTA and 10% glycerol) containing protease inhibitors (10 μg/ml each of aprotinin and leupeptin and 0.1 mM phenylmethylsulphonyl fluoride) and phosphatase inhibitors (1 mM NaF, 0.1 mM Na3VO4 and 10 mM β-glycerophosphate). HA-tagged PKC proteins from 500 μg of cell extracts were immunoprecipitated with 3 μg of anti-HA antibody and 30 μl of protein G-Sepharose for 3 h at 4°C. The immunoprecipitates were resuspended in PKC reaction buffer (50 mM HEPES (pH 7.5), 10 mM MgCl2, 1 mM dithiothreitol, 2.5 mM EGTA, 1 mM NaF, 0.1 mM Na3VO4 and 10 mM β-glycerophosphate). The kinase assay was initiated by adding PKC reaction buffer containing 5 μg of PKC-ɛ substrate and 5 μCi of [γ-32P]ATP. Reactions were carried out for 30 min at 37°C and terminated by adding sodium dodecyl sulfate (SDS) sample buffer. Mixtures were then boiled for 5 min, and the reaction products were analyzed by SDS–polyacrylamide gel electrophoresis (PAGE) and autoradiography.
Statistical analysis
All data were expressed as mean±s.d. The numerical data were compared using a one-way analysis of variance followed by a post-test such as Bonferoni test. A P-value of <0.05 was considered significant.
Antibodies and drugs
Myristoylated PKC ɛV1–2 (ɛV1–2; an inhibitor of PKC-ɛ) was purchased from Biomol Research Lab (Plymouth Meeting, PA, USA). Diazoxide, 5-hydroxydecanoate (5-HD; a specific inhibitor of mitoKATP channel), cyclosporin A (CsA; a permeability transition pore (PTP) inhibitor) and DNP (metabolic uncoupler) were purchased from Sigma (St Louis, MO, USA). Ruthenium Red (RR; an inhibitor of mitochondrial Ca2+ uniporter) was obtained from TOCRIS (Ellisville, MO, USA). Anti-PKC-α, -βI, -δ, -ɛ, -ζ (1:500) and anti-HA (1:500) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-actin (1:3000) and anti-DRP (1:1000) antibodies were from Sigma (St Louis, Mo, USA). Anti-COX (1:3000) antibody was from Molecular Probes (Eugene, OR, USA). Diazoxide, ɛV1–2, CsA, and RR were dissolved in dimethyl sulfoxide (DMSO) to form stock solutions. The final DMSO concentration of 0.1% was found to have no effect on neonatal rat cardiomyocytes and H9c2 cell viability. The concentrations of inhibitors and treatments were chosen on the basis of preliminary studies (data not shown).
Results
Effect of diazoxide on PKC isoforms
Figures 1a and 2a show isoform-specific translocation of PKC-ɛ by diazoxide in H9c2 and neonatal rat cardiomyocytes, respectively. In both cells, the level of PKC-ɛ in the particulate fraction started to increase as early as 5 min and reached a peak at 30 min after treatment with diazoxide (100 μM). The level of PKC-ɛ in the soluble fraction was markedly decreased. The other PKC isoforms (PKC-α, -βI, -δ, -ζ) were not altered by diazoxide treatment in either the particulate or the soluble fractions. To evaluate whether diazoxide-induced translocation of PKC-ɛ involves mitochondrial translocation, we evaluated protein expression of PKC-ɛ in the mitochondrial fraction as well as the cytosolic fraction. As shown in Figures 1b and 2b, the cellular subfractions, in terms of the mitochondrial, as well as the cytosolic and the plasma membrane fractions, were characterized by their specific markers, COX, actin and DRP, and no cross-contamination was found between the cellular fractions. The level of PKC-ɛ in the mitochondrial fraction increased at 5 min after 100 μM diazoxide treatment while levels in the cytosolic fractions were decreased remarkably (Figures 1b and c, 2b and c). The activity of PKC-ɛ in the mitochondrial fraction began to increase as early as 5 min after diazoxide treatment, with a maximal increase after 10 min, in H9c2 cells and neonatal rat cardiomyocytes (Figures 1d and 2d).
Figure 1.
Effect of diazoxide (DZX) on PKC isotypes in H9c2 cells. (a) Western blots for PKC isotypes (-α, -βI, -δ, -ɛ and -ζ) detected in the soluble and the particulate fraction after various periods of diazoxide treatment. Data shown are representative of four separate experiments. COX, cytochrome c oxidase subunit IV. (b) Western blots for PKC-ɛ detected in cytosolic and the mitochondrial fraction after various periods of diazoxide treatment (lower panel). Western blots for subcellular markers, cytochrome c oxidase subunit IV (COX), dihydropyridine receptor protein (DRP) and actin (upper panel). Data shown are representative of four separate experiments. Cyt, cytosol; Mt, mitochondria; Pm, plasma membrane. (c) Quantitative analysis for PKC-ɛ in the cytosolic and the mitochondrial fraction after various periods of diazoxide treatment. Data shown as means±s.d. (n=4) represent the percentage of the control (CTL, 0 time). *P<0.05 vs vehicle-treated control (CTL, 0 time). (d) Kinase activity for PKC-ɛ measured in mitochondrial fraction after various periods of diazoxide treatment. Data shown as means±s.d. (n=4) represent the percentage of the control (CTL, 0 time). *P<0.05 vs vehicle-treated control (CTL, 0 time).
Figure 2.
Effect of diazoxide (DZX) on PKC isotypes in neonatal rat cardiomyocytes. (a) Western blots for PKC isotypes (-α, -βI, -δ, -ɛ and -ζ) detected in the soluble and the particulate fraction after various periods of diazoxide treatment. Data shown are representative of four separate experiments. COX, cytochrome c oxidase subunit IV. (b) Western blots for PKC-ɛ detected in cytosolic and the mitochondrial fraction after various periods of diazoxide treatment (lower panel). Western blots for subcellular markers, cytochrome c oxidase subunit IV (COX), dihydropyridine receptor protein (DRP) and actin (upper panel). Data shown are representative of four separate experiments. Cyt, cytosol; Mt, mitochondria; Pm, plasma membrane. (c) Quantitative analysis for PKC-ɛ in the cytosolic and the mitochondrial fraction after various periods of diazoxide treatment. Data shown as means±s.d. (n=4) represent the percentage of the control (CTL, 0 time). *P<0.05 vs vehicle-treated control (CTL, 0 time). (d) Kinase activity for PKC-ɛ measured in mitochondrial fraction after various periods of diazoxide treatment. Data shown as means±s.d. (n=4) represent the percentage of the control (CTL, 0 time). *P<0.05 vs vehicle-treated control (CTL, 0 time).
The role of PKC-ɛ in diazoxide-induced cardioprotection against apoptotic cell death during hypoxia
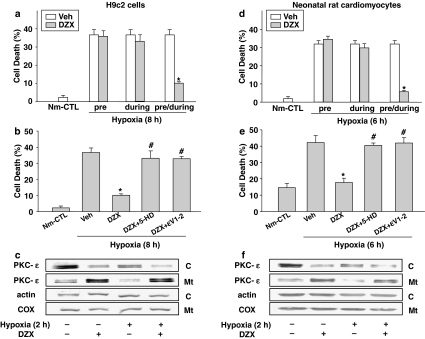
To investigate the role of translocated PKC-ɛ to the mitochondria induced by diazoxide, we examined the effect of specific blockade of PKC-ɛ translocation by ɛV1–2 on apoptotic cell death after 8 h of hypoxia in both H9c2 cells (Figure 3a and b) and 6 h of hypoxia in neonatal rat cardiomyocytes (Figure 3d and e). Periods of hypoxia, producing about 50% of cell death, were determined by preliminary experiment (data not shown), and differences between the two cell types appears due to the greater tendency of the neonatal rat cardiomyocytes to detach from the culture plate and their shorter time course of cell death compared to H9c2 cells. The proportion of TUNEL-positive cells after 8 h of hypoxia in H9c2 cells, and that after 6 h of hypoxia in neonatal rat cardiomyocytes was the same, about 30–35% (Figure 3a and d). The TUNEL-positive cells in both cultures were attenuated by diazoxide treatment in the ‘pre/during' group, but not in the ‘pre' nor in the ‘during' group. The anti-apoptotic effect of diazoxide (Figure 3b and e) was almost completely blocked by 10 μM ɛV1–2 and by 100 μM 5-HD. These results suggest the involvement of PKC-ɛ as well as mitoKATP channel in the mechanism of diazoxide-induced cardioprotection. Consistent with these results, Figure 3c (H9c2 cells) and f (neonatal rat cardiomyocytes) show that a high level of PKC-ɛ in the mitochondrial fraction of diazoxide-treated group was maintained, up to 2 h of hypoxia, while the protein levels of PKC-ɛ in both the cytosolic and the mitochondrial fraction (Kim et al., 2003) were diminished after 2 h of hypoxia in the hypoxic control cultures.
Figure 3.
The role of PKC-ɛ in diazoxide-induced cardioprotection against hypoxia-induced apoptotic cell death in H9c2 cells (a–c) and in neonatal rat cardiomyocytes (d–f). (a and d) Effect of diazoxide (DZX) on hypoxia-induced apoptotic cell death. The cells were treated with 100 μM diazoxide 1 h before hypoxia (‘pre'), during hypoxia (‘during') or 1 h before and during hypoxia (‘pre/during'). Cell death (%) was calculated by dividing the number of TUNEL-stained cells by total number of cells 8 h (a) and 6 h (d) after hypoxic insult. The data are mean±s.d. (n=5). *P<0.05 vs vehicle-treated hypoxic control (Veh). Nm-CTL, vehicle-treated normoxic control. (b and e) Effect of PKC-ɛ inhibitor (ɛV1–2) and mitoKATP channel inhibitor (5-HD) on diazoxide-induced cardioprotection against hypoxia-induced apoptotic cell death. The cells were treated with 100 μM diazoxide 1 h before and during hypoxia in the absence or presence of 10 μM ɛV1–2 or 100 μM 5-HD. Cell death (%) was calculated by dividing the number of TUNEL-stained cells by total number of cells 8 h (b) and 6 h (e) after hypoxic insult. The data are mean±s.d. (n=5). *P<0.05 vs vehicle-treated hypoxic control (Veh), #P<0.05 vs diazoxide-treated hypoxia (DZX). Nm-CTL, vehicle-treated normoxic control. (c and f) Effect of diazoxide on PKC-ɛ expression during hypoxia. The cells were treated with 100 μM diazoxide for 1 h before and during hypoxia. Western blots for PKC-ɛ detected in the cytosolic (C) and the mitochondrial (Mt) fractions after 2 h of hypoxia. Data shown are representative of four separate experiments. COX, cytochrome c oxidase subunit IV.
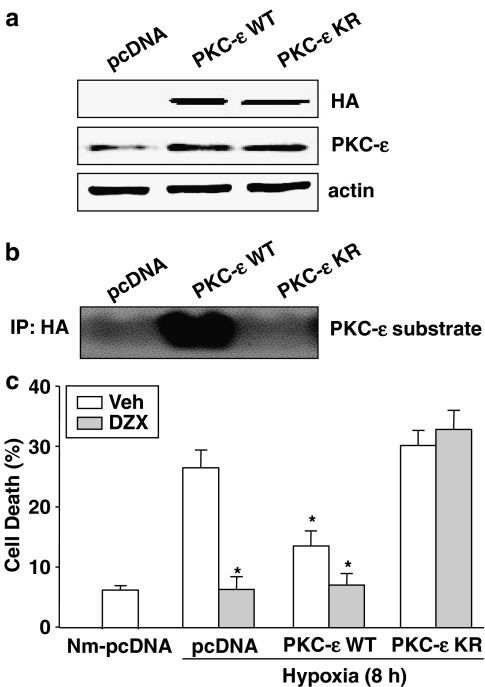
As shown in Figure 4a, the expression vectors for PKC-ɛ WT or PKC-ɛ KR were transfected into H9c2 cells, to verify that they expressed the predicted protein (HA), and the constructs were found to express the corresponding proteins with expected sizes at comparable levels. In addition, increased kinase activity of PKC-ɛ was found in PKC-ɛ WT transfected cells and decreased PKC-ɛ kinase activity was shown in PKC-ɛ KR transfected cells (Figure 4b). As shown in Figure 4c, in cells transfected with pcDNA3, about 28% was shown as TUNEL-positive after 8 h of hypoxia. This increase in TUNEL-positive cells was dramatically attenuated by diazoxide treatment or by transfection of PKC-ɛ WT. The anti-apoptotic effect of diazoxide during hypoxia was almost completely blocked in PKC-ɛ KR transfected cells.
Figure 4.
The role of PKC-ɛ in diazoxide-induced cardioprotection against hypoxia-induced apoptotic cell death in PKC-ɛ transfected cells. H9c2 cells were transfected with PKC-ɛ sequence (PKC-ɛ WT or PKC-ɛ KR) vector and control vector pcDNA3. (a) Western blots for HA or PKC-ɛ detected in the PKC-ɛ transfected cells. Data shown are representative of four separate experiments. (b) Kinase activity for PKC-ɛ measured in the PKC-ɛ transfected cells. Data shown are representative of four separate experiments. (c) The role of PKC-ɛ in diazoxide-induced cardioprotection against hypoxia-induced apoptotic cell death. The PKC-ɛ transfected cells were treated with or without 100 μM diazoxide (DZX) 1 h before and during hypoxia. Cell death (%) was calculated by dividing the number of TUNEL-stained cells by total number of cells 8 h after hypoxic insult. The data are mean±s.d. (n=4). *P<0.05 vs vehicle-pcDNA, Nm, normoxia.
Link between PKC-ɛ and the mitoKATP channel
To investigate the link between PKC-ɛ and the mitoKATP channel, we examined the effect of 5-HD on PKC-ɛ translocation and the effect of ɛV1–2 on mitoKATP channel activation, monitored by flavoprotein oxidation. Opening of mitoKATP channels dissipates the inner Δψm established by the proton pump. This dissipation accelerates electron transfer by the respiratory chain and if uncompensated by increased production of electron donors, leads to net oxidation of the mitochondria. We therefore monitored the mitochondrial redox state by recording the fluorescence of flavoprotein in the mitochondria as an indirect index of the mitoKATP channel opening (Liu et al., 1998; Wang et al., 2001a). Figures 5a and 6a show the time course of changes in flavoprotein fluorescence in H9c2 cells and neonatal rat cardiomyocytes exposed to diazoxide. Flavoprotein fluorescence began to increase as early as 7 min after diazoxide treatment, with a resultant plateau at about 10 min. Consistent with earlier reports (Liu et al., 1998), these diazoxide-induced flavoprotein oxidations were completely inhibited by 5-HD to control level in both types of cell. As shown in Figures 5b and 6b, the maximum flavoprotein oxidations by diazoxide were completely blocked by 10 μM ɛV1–2. When we investigated the effect of 5-HD on PKC-ɛ translocation, diazoxide-induced PKC-ɛ translocation to mitochondria remained unaltered after 5-HD treatment in both H9c2 cells and neonatal rat cardiomyocytes (Figures 5c and 6c). The kinase activity of PKC-ɛ in the mitochondrial fraction was almost doubled by treatment with diazoxide (Figures 5d and 6d). This increase was blocked by 10 μM ɛV1–2 but not by 5-HD, in both H9c2 cells and neonatal rat cardiomyocytes.
Figure 5.
Link between PKC-ɛ and the mitoKATP channel in H9c2 cells. The cells were treated with 100 μM diazoxide (DZX) in the absence or presence of PKC-ɛ inhibitor (ɛV1–2) or mitoKATP channel inhibitor (5-HD). (a) Effect of 10 μM ɛV1–2 and 100 μM 5-HD on diazoxide-induced flavoprotein (FP) oxidation. FP oxidation (%) was calculated as a percentage of the 100 μM DNP-induced fluorescence. Data shown are representative of four separate experiments. (b) Effect of 10 μM ɛV1–2 and 100 μM 5-HD on diazoxide-induced maximum flavoprotein (FP) oxidation. FP oxidation (%) was calculated as a percentage of the 100 μM DNP-induced fluorescence. The data are mean±s.d. (n=4). *P<0.05 vs control (CTL), #P<0.05 vs diazoxide alone (Veh). (c) Effect of 10 μM ɛV1–2 and 100 μM 5-HD on diazoxide-induced translocation of PKC-ɛ. Western blots for PKC-ɛ detected in the cytosolic (C) and the mitochondrial (Mt) fractions after 10 min of diazoxide. Data shown are representative of four separate experiments. COX, cytochrome c oxidase subunit IV. (d) Effect of 10 μM ɛV1–2 and 100 μM 5-HD on diazoxide-induced increase of kinase activity of PKC-ɛ in mitochondria. The data are mean±s.d. (n=4). *P<0.05 vs vehicle-treated control (CTL), #P<0.05 vs DZX alone.
Figure 6.
Link between PKC-ɛ and the mitoKATP channel in neonatal rat cardiomyocytes. The cells were treated with 100 μM diazoxide (DZX) in the absence or presence of PKC-ɛ inhibitor (ɛV1–2) or mitoKATP channel inhibitor (5-HD). (a) Effect of 10 μM ɛV1–2 and 100 μM 5-HD on diazoxide-induced flavoprotein (FP) oxidation. FP oxidation (%) was calculated as a percentage of the 100 μM DNP-induced fluorescence. Data shown are representative of four separate experiments. (b) Effect of 10 μM ɛV1–2 and 100 μM 5-HD on diazoxide-induced maximum flavoprotein (FP) oxidation. FP oxidation (%) was calculated as a percentage of the 100 μM DNP-induced fluorescence. The data are mean±s.d. (n=4). *P<0.05 vs control (CTL), #P<0.05 vs diazoxide alone (Veh). (c) Effect of 10 μM ɛV1–2 and 100 μM 5-HD on diazoxide-induced translocation of PKC-ɛ. Western blots for PKC-ɛ detected in the cytosolic (C) and the mitochondrial (Mt) fractions after 10 min of diazoxide. Data shown are representative of four separate experiments. COX, cytochrome c oxidase subunit IV. (d) Effect of 10 μM ɛV1–2 and 100 μM 5-HD on diazoxide-induced increase of kinase activity of PKC-ɛ in mitochondria. The data are mean±s.d. (n=4). *P<0.05 vs vehicle-treated control (CTL), #P<0.05 vs DZX alone.
On the basis of these results, the role of specific translocation of PKC-ɛ by diazoxide in hypoxic cell death and the interrelationship between PKC-ɛ and the mitoKATP channel were shown to be quite similar each other in both H9c2 cells and neonatal rat cardiomyocytes. We therefore performed the following experiments using only the more convenient H9c2 cells.
Effect of PKC-ɛ on mitoKATP channel opening in PKC-ɛ transfected cells
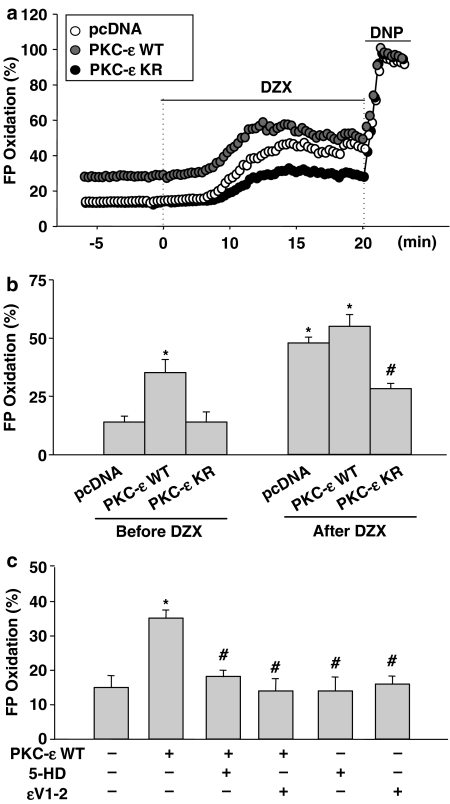
In order to confirm the link between PKC-ɛ and mitoKATP channel, we further examined the effect of direct PKC-ɛ activation or inactivation on flavoprotein oxidation, with H9c2 cells transfected with PKC-ɛ WT or PKC-ɛ KR. In cells transfected with PKC-ɛ WT, flavoprotein fluorescence was increased before diazoxide treatment and increased further after diazoxide treatment (Figure 7a and b). Maximum flavoprotein oxidation induced by diazoxide was markedly blocked in cells transfected with the negative mutant, PKC-ɛ KR (Figure 7a and b). Figure 7c shows that overexpression of PKC-ɛ WT-induced increase in flavoprotein oxidation was completely blocked by 5-HD, as well as by ɛV1–2.
Figure 7.
Link between PKC-ɛ and the mitoKATP channel in PKC-ɛ transfected cells. The H9c2 cells were transfected with PKC-ɛ sequence (PKC-ɛ WT and PKC-ɛ KR) vector and control vector pcDNA3. (a) Effect of transfection of PKC-ɛ on diazoxide-induced flavoprotein (FP) oxidation. The PKC-ɛ transfected cells were treated with or without 100 μM diazoxide (DZX). FP oxidation (%) was calculated as a percentage of the 100 μM DNP-induced fluorescence. Data shown are representative of four separate experiments. (b) Effect of transfection of PKC-ɛ on diazoxide-induced maximum flavoprotein (FP) oxidation. The PKC-ɛ transfected cells were treated with or without 100 μM diazoxide. FP oxidation (%) was calculated as a percentage of the 100 μM DNP-induced fluorescence. The data are mean±s.d. (n=4). *P<0.05 vs pcDNA alone, #P<0.05 vs diazoxide-treated pcDNA. (c) Effect of PKC-ɛ inhibitor (ɛV1–2) and mitoKATP channel inhibitor (5-HD) on PKC-ɛ overexpression-induced maximum flavoprotein (FP) oxidation. The PKC-ɛ transfected cells were treated with 100 μM diazoxide in the absence or presence of 10 μM ɛV1–2 or 100 μM 5-HD. The data are mean±s.d. (n=4). *P<0.05 vs vehicle-treated PKC-ɛ WT, #P<0.05 vs PKC-ɛ WT.
The role of PKC-ɛ in diazoxide-induced inhibitory effect on mitochondrial death pathway
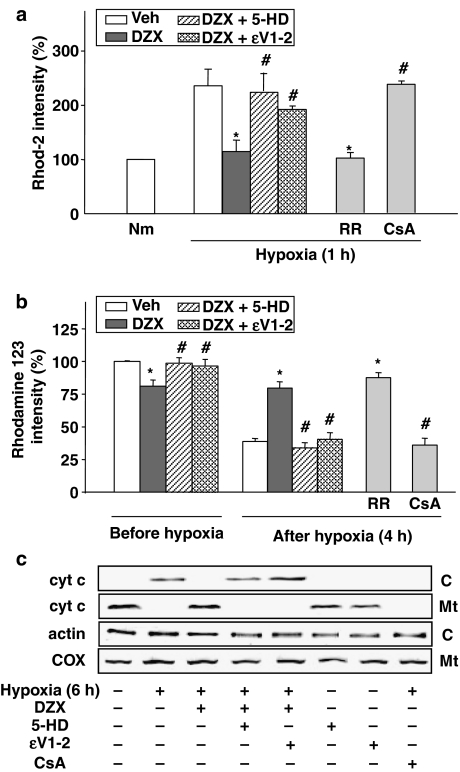
To elucidate the role of the PKC-ɛ in mitochondrial death pathway during hypoxia, we measured [Ca2+]mito, Δψm and cytochrome c release from the mitochondria to the cytosol during hypoxia in H9c2 cells. As demonstrated in our previous results, hypoxia-induced elevation of [Ca2+]mito, and disruption of Δψm reaches peak at 1 and 4 h of hypoxia, respectively, and the level of cytochrome c released into cytosol reaches peak at 6 h of hypoxia (Kim et al., 2005). Hypoxia-induced [Ca2+]mito elevation and Δψm reduction was almost completely blocked by RR, but not by CsA (Figure 8a and b), suggesting that hypoxia-induced Δψm reduction is dependent on [Ca2+]mito elevation rather than PTP opening. As expected, hypoxia-induced cytochrome c release was inhibited by CsA. Figure 8b shows that diazoxide-induced reduction of Δψm was completely abolished by 10 μM ɛV1–2 to control levels (Veh). As shown in Figure 8a–c, the treatment with diazoxide prevented the increase in [Ca2+]mito, mitochondrial depolarization and cytochrome c release induced by hypoxia, and all these effects of diazoxide were almost completely blocked by ɛV1–2 as well as 5-HD.
Figure 8.
The role of PKC-ɛ in diazoxide-induced inhibitory effect on mitochondrial death pathway. The cells were treated with 100 μM diazoxide (DZX) for 1 h before and during hypoxia in the absence or presence of PKC-ɛ inhibitor (ɛV1–2) or KATP channel inhibitor (5-HD). (a) Effect of 10 μM ɛV1–2 and 100 μM 5-HD on diazoxide-induced attenuation of mitochondrial Ca2+ ([Ca2+]mito) overload during hypoxia. Quantitative analysis of rhod-2 intensity after 1 h of hypoxia. Data shown as means±s.d. (n=5) represent the percentage of the vehicle-treated normoxic control (Nm). *P<0.05 vs vehicle-treated hypoxic control (Veh). #P<0.05 vs diazoxide alone (DZX). RR, Ruthenium Red; CsA, cyclosporin A. (b) Effect of 10 μM ɛV1–2 and 100 μM 5-HD on reduction of mitochondrial membrane potential (Δψm). Δψm was determined by FACScan analysis of rhodamine 123 intensity after 4 h of hypoxia. Data shown as means±s.d. (n=4) represent the percentage of the vehicle-treated normoxic control. #P<0.05 vs vehicle-treated control (Veh). *P<0.05 vs DZX alone. RR, Ruthenium Red; CsA, cyclosporin A. (c) Effect of 10 μM ɛV1–2 and 100 μM 5-HD on diazoxide-induced cytochrome c release during hypoxia. Western blots for cytochrome c detected in the cytosolic (C) and the mitochondrial (Mt) fractions after 6 h of hypoxia. Blots shown are representative of four separate experiments. CsA, cyclosporin A; COX, cytochrome c oxidase subunit IV.
Discussion
The main findings of this study are that diazoxide-induced isoform-specific activation of PKC-ɛ is an intermediate step in the opening of the mitoKATP channel, resulting in cardioprotection against hypoxia-induced death in H9c2 cells and neonatal rat cardiomyocytes.
This study demonstrates that diazoxide induces isoform-specific translocation of PKC-ɛ from the soluble to the particulate, especially to the mitochondrial fraction, and that diazoxide increases PKC-ɛ activity in the mitochondrial fraction in H9c2 cell lines and primary culture of neonatal rat cardiomyocytes (Figures 1 and 2). This study further shows that measurable protein levels and activities of PKC-ɛ can be detected in the mitochondrial fraction in the absence of diazoxide (Figures 1d and 2d), suggesting the existence of PKC-ɛ inside mitochondria in the basal state. Consistent with our results, recent reports demonstrated that PKC-ɛ exists constitutively inside mitochondria, but only equivalent to 10–20% of that observed in the cytosol in the basal state and that PKC-ɛ is either very tightly bound or, more likely, is localized in the intermembrane space in heart mitchondria (Baines et al., 2002; Costa et al., 2005).
The results obtained from the pharmacological and the molecular approach using ɛV1–2, and a dominant-negative mutant form of PKC-ɛ, suggest the essential role of PKC-ɛ activation in diazoxide-induced cardioprotection (Figures 3b, e and 4c). Although these results are not consistent with previous studies in rat heart, suggesting that diazoxide-induced translocation of PKC-δ is associated with cardioprotection (Wang and Ashraf, 1999; Wang et al., 2001b), there are reports consistent with ours, suggesting a role for PKC-ɛ in diazoxide-induced cardioprotection in chick cardiomyocytes (Liu et al., 2002). Other pharmacological actions of diazoxide have been known to include inhibition of succinate dehydrogenase (SDH) (Schafer et al., 1969); the present study, however, did not consider the contribution of SDH inhibition to diazoxide-induced cardioprotection, because the concentration of diazoxide used in our study was much lower (100 μM) than that needed to achieve SDH inhibition (∼400 μM) (Schafer et al., 1969; Grimmsmann and Rustenbeck, 1998).
As in the case of our previous study with KR-31378, a novel KATP opener (Moon et al., 2004), the cardioprotective effect of diazoxide was observed only in the ‘pre/during' group and not in the ‘pre' or the ‘during' group (Figure 3a and d). These results are inconsistent with other studies showing that cardioprotective efficacy was still demonstrable when diazoxide treatment was commenced during reperfusion (Hausenloy et al., 2004) or only before exposure to H2O2 (Akao et al., 2003). These discrepancies cannot be readily explained, but may be due to different environments between in vivo and in vitro systems, and injury types.
From our results that ‘pre/during' treatment with diazoxide exerts cardioprotection, involving PKC-ɛ and the mitoKATP channel, we would suggest that the modulation of PKC-ɛ and the mitoKATP channel by diazoxide should precede hypoxic insult and be maintained during hypoxia. These results prompted us to investigate further the relationship between PKC-ɛ activation and the mitoKATP channel opening, in the presence of diazoxide, before hypoxia. Our findings showed that blockade of PKC-ɛ by ɛV1–2 or dominant-negative mutant form of PKC-ɛ inhibited diazoxide-induced flavoprotein oxidation (Figure 3b and e), while diazoxide-induced PKC-ɛ translocation and activation remained unaltered after blockade of the mitoKATP channel by 5-HD (Figure 3b–d and f). Based on these results, we would propose two possible signaling schemes: one, in which the signaling pathways for diazoxide-induced mitoKATP channel opening and PKC-ɛ activation are independent of each other, and the other, in which diazoxide-induced PKC-ɛ activation occurs upstream of the activation of the mitoKATP channel. Although we cannot exclude the possibility of independent signaling pathways for PKC-ɛ and the mitoKATP channel, several pieces of evidence favor the latter, sequential, pathway. First, because either ɛV1–2 or 5-HD could completely, rather than partially, block the flavoprotein oxidizing effect of diazoxide (Figures 5a,b and 6a,b), it is likely that diazoxide-induced PKC-ɛ activation and mitoKATP channel-dependent flavoprotein oxidation share the same signaling pathway. The partial inhibitory effect of transfection with PKC-ɛ KR mutant on the flavoprotein oxidizing effect of diazoxide may be due to the contribution of endogenous normal PKC-ɛ in the presence of diazoxide (Figure 7a and b). Second, direct activation of PKCs by overexpression of PKC-ɛ WT mimicked the flavoprotein oxidizing effect of diazoxide (Figure 7a), which was completely blocked by 5-HD, suggesting a signaling cascades of: PKC-ɛ → mitoKATP channel → flavoprotein oxidation. Third, as judged from the time course results, because diazoxide-induced PKC-ɛ translocation reached its maximum level at 5 min (Figures 1 and 2) and the corresponding flavoprotein oxidation was maximal at about 10 min (Figures 5a and 6a), it is likely that mitoKATP channel-dependent flavoprotein oxidation occurs downstream of PKC-ɛ activation. Taken together, these considerations lead us to suggest that diazoxide first induces isoform-specific translocation of PKC-ɛ into mitochondria, which in turn activates the mitoKATP channel located on the inner mitochondrial membrane. Translocation of the cytosolic PKC-ɛ to the mitochondria by diazoxide seems to be essential for PKC-ɛ to link with the mitoKATP channel, leading to the mitoKATP channel opening. In addition, mitochondrial localization of PKC-ɛ in the basal state provides another possibility for PKC-ɛ to link with the mitoKATP channel in the presence of diazoxide, leading to the mitoKATP channel opening.
It has been known that during hypoxia, cytosolic Ca2+ increases and mitochondria takes up some of the increased cytosolic Ca2+ through a RR-sensitive Ca2+ uniporter (Anderson et al., 2005), which in turn induces the opening of PTP, and the disruption of the Δψm, leading to release of cytochrome c, caspase-3 activation and apoptotic cell death (Gunter et al., 1994). Supporting this notion, our study confirmed that hypoxia-induced [Ca2+]mito elevation and Δψm reduction were almost completely blocked by RR (Figure 8a and b). From the results using CsA, an inhibitor of PTP opening (Figure 7a and b), it is suggested that hypoxia-induced disruption of Δψm may not be caused by PTP in H9c2 cells. As demonstrated in our previous results, hypoxia-induced disruption of Δψm reaches a peak at 4 h of hypoxia (Kim et al., 2005) and the cytochrome c release into cytosol reaches a peak at 6 h of hypoxia (Figure 8c). From these results, it is suggested that the disruption of Δψm by hypoxia-induced [Ca2+]mito overload may cause PTP opening and subsequently cytochrome c release in cardiomyocytes. Mitochondrial Ca2+ overload and the large reduction in Δψm during hypoxia were markedly attenuated by diazoxide in the ‘pre/during' treatment group (Figure 8a and b). Taking into consideration that the driving force responsible for the mitochondrial Ca2+ uptake through the Ca2+ uniporter is the difference in Δψm across the inner mitochondrial membrane (Smaili et al., 2003), the present results strongly support the previous notion that mitoKATP channel opening by diazoxide partially depolarizes Δψm before hypoxia and thus might attenuate the massive overload of mitochondrial Ca2+ during hypoxia by decreasing the driving force for mitochondrial Ca2+ uptake (Holmuhamedov et al., 1998). Our results further demonstrated that inhibitory effects of diazoxide on hypoxia-induced increase in [Ca2+]mito and reduction in Δψm, as well as hypoxia-induced cytochrome c release and cell death, were almost completely abolished by ɛV1–2 as well as 5-HD. These results indicate that diazoxide-induced cardioprotection involving modulation of the mitochondrial death pathway is mediated through activation of PKC-ɛ as well as the mitoKATP channel opening, although further studies on the mitochondrial death pathway need to be performed with neonatal rat cardiomyocytes because these results were obtained from H9c2 cells (Figure 8).
Taken together, the present results suggest a signaling pathway by which diazoxide induces mitoKATP channel opening mediated through PKC-ɛ activation, leading to cardioprotection. There have been several reports suggesting that PKC is an upstream signaling molecule of mitoKATP channel (Ohnuma et al., 2002; Hassouna et al., 2004), which appears to be similar to our results, at a first glance. These studies, however, were quite different from ours because they were carried out on the assumption that diazoxide is a direct activator of the mitoKATP channel, and suggested a signal transduction sequence of PKC → diazoxide-induced direct activation of the mitoKATP channel. On the other hand, our study suggests an indirect signaling pathway for the action of diazoxide on the mitoKATP channel opening, that is, diazoxide → PKC-ɛ → mitoKATP channel → Δψm reduction. Although it is not clear how PKC-ɛ translocation induces opening of the mitoKATP channel, it is possible that PKC-ɛ translocated to the mitochondria by diazoxide regulates activity of the mitoKATP channel, possibly by phosphorylation of a relevant protein (Light et al., 2000).
In summary, the results from this study suggest that diazoxide may be acting more as a PKC-ɛ activator than a direct mitoKATP channel opener in H9c2 cells lines and neonatal rat cardiomyocytes. Further studies should be investigated whether diazoxide can directly modulate the recombinant PKC-ɛ in vitro, which would add evidence for the potential use of diazoxide as a specific PKC-ɛ activator.
Acknowledgments
This study was supported by a grant (CBM2-A300-001-1-0-2) from the center for Biological Modulators of the 21st century Frontier R&D program, the Ministry of Science and Technology, and by a grant (R-01-2005-000-10510-0) from Basic Research Program of the Korea Science and Engineering Foundation.
Abbreviations
- Δψm
mitochondrial membrane potential
- [Ca2+]mito
mitochondrial Ca2+ level
- ɛV1–2
myristoylated PKC ɛV1–2
- 5-HD
5-hydroxydecanoate
- COX
cytochrome oxidase subunit IV
- CsA
cyclosporin A
- DMSO
dimethyl sulfoxide
- DNP
2,4-dinitrophenol
- DRP
dihydropyridine receptor protein
- I/R
ischemia/reperfusion
- mitoKATP
mitochondrial ATP-sensitive potassium channel
- RR
Ruthenium Red
Conflicts of interest
The authors state no conflict of interest.
References
- Akao M, O'rourke , Kusuoka H, Teshima Y, Jones SP, Marban E. Differential actions of cardioprotective agents on the mitochondrial death pathway. Circ Res. 2003;92:195–202. doi: 10.1161/01.res.0000051862.16691.f9. [DOI] [PubMed] [Google Scholar]
- Anderson CD, Pierce J, Nicoud I, Belous A, Knox CD, Chari RS. Modulation of mitochondrial calcium management attenuates hepatic warm ischemia-reperfusion injury. Liver Transpl. 2005;11:663–668. doi: 10.1002/lt.20407. [DOI] [PubMed] [Google Scholar]
- Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, et al. Mitochondrial PKCɛ and MAPK from signaling modules in the murine heart. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, et al. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- Disatnik MH, Isatnik MH, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res. 1994;210:287–297. doi: 10.1006/excr.1994.1041. [DOI] [PubMed] [Google Scholar]
- Emaus RK, Grunwald R, Lemasters JJ. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986;850:436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- Grimmsmann T, Rustenbeck I. Direct effects of diazoxide on mitochondria in pancreatic B-cells and on isolated liver mitochondria. Br J Pharmacol. 1998;123:781–788. doi: 10.1038/sj.bjp.0701663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Hassouna A, Matata BM, Galinanes M. PKC-ɛ is upstream and PKC-á is downstream of mitoKATP channels in the signal transduction pathway of ischemic preconditioning of human myocardium. Am J Physiol Cell Physiol. 2004;287:C1418–C1425. doi: 10.1152/ajpcell.00144.2004. [DOI] [PubMed] [Google Scholar]
- Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- Holmuhamedov EL, Jovanovic S, Dzeja PP, Jovanovic A, Terzic A. Mitochondrial ATP-sensitive K+ channels modulate cardiac mitochondrial function. Am J Physiol. 1998;275:H1567–H1576. doi: 10.1152/ajpheart.1998.275.5.H1567. [DOI] [PubMed] [Google Scholar]
- Jung YS, Jung YS, Kim MY, Kim MH, Lee S, Yi KY, et al. KR-31466, a benzopyranylindol analog, attenuates hypoxic injury through mitochondrial KATP channel and protein kinase C activation in heart-derived H9c2 cells. J Pharmacol Sci. 2003;92:13–18. doi: 10.1254/jphs.92.13. [DOI] [PubMed] [Google Scholar]
- Kim MH, Jung YS, Moon CH, Jeong EM, Lee SH, Baik EJ, et al. Isoform-specific induction of PKC-ɛ by high glucose protects heart-derived H9c2 cells against hypoxic injury. Biochem Biophys Res Commun. 2003;309:1–6. doi: 10.1016/s0006-291x(03)01525-0. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Moon CH, Kim MY, Lee S, Yi KY, Yoo SE, et al. KR-32570, a novel Na+/H+ exchanger-1 inhibitor, attenuates hypoxia-induced cell death through inhibition of intracellular Ca2+ overload and mitochondrial death pathway in H9c2 cells. Eur J Pharmacol. 2005;525:1–7. doi: 10.1016/j.ejphar.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Soh JW, Jeoung DI, Cho CK Jhon GJ, Lee SJ, Lee YS. PKC-ɛ-mediated ERK1/2 activation involved in radiation-induced cell death in NIH3T3 cells. Biochim Biophys Acta. 2003;1593 2–3:219–229. doi: 10.1016/s0167-4889(02)00392-0. [DOI] [PubMed] [Google Scholar]
- Light PE, Bladen C, Winkfein RJ, Walsh MP, French RJ. Molecular basis of protein kinase C-induced activation of ATP-sensitive potassium channels. Proc Natl Acad Sci USA. 2000;97 16:9058–9063. doi: 10.1073/pnas.160068997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sato T, O'rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang HY, Zhu X, Shao Z, Yao Z. Preconditioning blocks cardiocyte apoptosis: role of KATP channels and PKC-ɛ. Am J Physiol Heart Circ Physiol. 2002;282:H1380–H1386. doi: 10.1152/ajpheart.00348.2001. [DOI] [PubMed] [Google Scholar]
- Moon CH, Kim MY, Kim MJ, Kim MH, Lee S, Yi KY, et al. KR-31378, a novel benzopyran analog, attenuates hypoxia-induced cell death via mitochondrial KATP channel and protein kinase C-ɛ in heart-derived H9c2 cells. Eur J Pharmacol. 2004;506:27–35. doi: 10.1016/j.ejphar.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Murata M, Akao M, O'rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca2+ overload during simulated ischemia and reperfusion: possible mechanism of cardioprotection. Circ Res. 2001;89:891–898. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- Ohnuma Y, Miura T, Miki T, Tanno M, Kuno A, Tsuchida A, et al. Opening of mitochondrial KATP channel occurs downstream of PKC-ɛ activation in the mechanism of preconditioning. Am J Physiol Heart Circ Physiol. 2002;283:H440–H447. doi: 10.1152/ajpheart.00434.2001. [DOI] [PubMed] [Google Scholar]
- Sato T, O'rourke B, Marban E. Modulation of mitochondrial ATP-dependent K+ channels by protein kinase C. Circ Res. 1998;83:110–114. doi: 10.1161/01.res.83.1.110. [DOI] [PubMed] [Google Scholar]
- Schafer G, Wegener C, Portenhauser R, Bojanovski D. Diazoxide, an inhibitor of succinate oxidation. Biochem Pharmacol. 1969;18:2678–2681. [PubMed] [Google Scholar]
- Simkhovich BZ, Przyklenk k, Kloner RA. Role of protein kinase C as a cellular mediator of ischemic preconditioning: a critical review. Cardiovasc Res. 1998;40:9–22. doi: 10.1016/s0008-6363(98)00142-4. [DOI] [PubMed] [Google Scholar]
- Smaili SS, Hsu YT, Carvalho AC, Rosenstock TR, Sharpe JC, Youle RJ. Mitochondria, calcium and pro-apoptotic proteins as mediators in cell death signaling. Braz J Med Biol Res. 2003;36:183–190. doi: 10.1590/s0100-879x2003000200004. [DOI] [PubMed] [Google Scholar]
- Trollinger DR, Cascio WE, Lemasters JJ. Selective loading of Rhod 2 into mitochondria shows mitochondrial Ca2+ transients during the contractile cycle in adult rabbit cardiac myocytes. Biochem Biophys Res Commun. 1997;236:738–742. doi: 10.1006/bbrc.1997.7042. [DOI] [PubMed] [Google Scholar]
- Wang S, Cone J, Liu Y. Dual roles of mitochondrial KATP channels in diazoxide-mediated protection in isolated rabbit hearts. Am J Physiol Heart Circ Physiol. 2001a;280:H246–H256. doi: 10.1152/ajpheart.2001.280.1.H246. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ashraf M. Role of protein kinase C in mitochondrial KATP channel-mediated protection against Ca2+ overload injury in rat myocardium. Circ Res. 1999;84:1156–1165. doi: 10.1161/01.res.84.10.1156. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirai K, Ashraf M. Activation of mitochondrial ATP-sensitive K+ channel for cardiac protection against ischemic injury is dependent on protein kinase C activity. Circ Res. 1999;85:731–741. doi: 10.1161/01.res.85.8.731. [DOI] [PubMed] [Google Scholar]
- Wang Y, Takashi E, Xu M, Ayub A, Ashraf M. Downregulation of protein kinase C inhibits activation of mitochondrial KATP channels by diazoxide. Circulation. 2001b;104:85–90. doi: 10.1161/01.cir.104.1.85. [DOI] [PubMed] [Google Scholar]
- Zaugg M, Lucchinetti E, Spahn DR, Pasch T, Garcia C, Schaub MC. Differential effects of anesthetics on mitochondrial KATP channel activity and cardiomyocyte protection. Anesthesiology. 2002;97:15–23. doi: 10.1097/00000542-200207000-00004. [DOI] [PubMed] [Google Scholar]