Abstract
Background and purpose:
Two metabolites of tryptophan, 5-hydroxyindole and kynurenic acid (kynurenate) affect the function of α7 nicotinic acetylcholine receptors (nAChRs), as measured by electrophysiological and Ca2+ fluorescence techniques. To better understand the modulations by 5-hydroxyindole and kynurenate of the function of nAChR subtypes, we compared the effects of 5-hydroxyindole and kynurenate on the release of various transmitters evoked by nAChR activation.
Experimental approach:
The function of α7nAChRs located on glutamatergic terminals was investigated by monitoring the release of [3H]D-aspartate or of endogenous glutamate from neocortical synaptosomes. We also comparatively considered non-α7 release-enhancing nAChRs localized on hippocampal noradrenergic or cholinergic terminals, as well as on striatal dopaminergic terminals.
Key results:
Epibatidine or nicotine, inactive on their own on basal release, enhanced [3H]D- aspartate and glutamate efflux in presence of 5-hydroxyindole. The release evoked by nicotine plus 5-hydroxyindole was abolished by methyllycaconitine or α-bungarotoxin. Presynaptic nAChRs mediating the release of [3H]noradrenaline ([3H]NA), [3H]dopamine ([3H]DA), or [3H]ACh were inhibited by 5-OHi. The α7nAChR-mediated release of [3H]D-aspartate was reduced by kynurenate at concentrations unable to affect the non-α7 receptor-mediated release of tritiated NA, DA or ACh.
Conclusions and Implications:
(i) 5-hydroxyindole permits selective activation of α7nAChRs mediating glutamate release; (ii) kynurenate down-regulates the permissive role of 5-hydroxyindole on α7nAChR activation; (iii) the non-α7nAChRs mediating release of NA, DA or ACh can be inhibited by 5-hydroxyindole, but not by kynurenate. These findings suggest up the possibility of developing novel drugs able to modulate selectively the cholinergic-glutamatergic transmission.
Keywords: nicotinic receptors, 5-hydroxyindole, kynurenic acid, glutamate release, catecholamine release, acetylcholine release
Introduction
Ionotropic neurotransmitter receptors possess allosteric binding sites, which mediate modulations of receptor function. Although the allosteric sites on GABAA receptors and glutamate NMDA receptors are well characterized, little is known about modulators of the function of nicotinic acetylcholine receptors (nAChRs), although a number of unconventional ligands and modulators of the nAChRs have been reported (Pereira et al., 2002; Hurst et al., 2005; Mok and Kew, 2006).
In the mammalian CNS, nAChRs have often been found to be localized on presynaptic terminals where they mediate neurotransmitter release (Wonnacott, 1997). Subtypes of presynaptic nAChRs consist of heteromeric or monomeric assemblies of various receptor subunits (Champtiaux et al., 2003; Gotti and Clementi, 2004). Receptors of the α7 subtype are abundant and widely distributed in the CNS; in particular, α7 nAChRs are localized on glutamatergic axon terminals where they mediate release of glutamate (McGehee et al., 1995; Marchi et al., 2002; Rousseau et al., 2005). Other nAChRs of various subunit compositions but lacking the α7 subunit (non- α7 nAChR), are present on different families of nerve endings where they modulate release of noradrenaline (NA), dopamine (DA) and ACh (Wonnacott, 1997 for a review).
Among the proposed nAChR modulators, 5-hydroxyindole and kynurenic acid, which are both metabolites of tryptophan (Moroni et al., 1988; Turski et al., 1988; Stone, 1993; Mannaioni et al., 2003), were found to affect the function of nAChRs of the α7 subtype. In particular, 5-hydroxyindole was able to potentiate currents and Ca2+ influx mediated by human α7 nAChRs expressed in Xenopus oocytes and GH4 cells, by α7 nAChRs expressed by IMR-32 cells, and by α7 nAChRs located in cerebellar mossy fibre–granule cell synapses (Zwart et al., 2002). Moreover, using electrophysiological and Ca2+ fluorescence techniques, Fucile et al. (2004) identified a subset of cultured cerebellar granule neurons that responded to nicotine through activation of multiple nAChRs. In a low percentage of cells, 5-hydroxyindole potentiated nicotine responses probably by activation of α7 nAChRs, whereas the remaining cells exhibited inhibition.
In contrast to the potentiating effects of 5-hydroxyindole, kynurenic acid was reported to inhibit electrophysiological responses mediated by α7 nAChRs. In particular, exposure of cultured hippocampal neurons to kynurenic acid caused inhibition of α7 nAChRs (Hilmas et al., 2001). Moreover, a targeted deletion of the kynurenine aminotransferase II gene which inhibited kynurenic acid biosynthesis, produced mice exhibiting, at immature age, electrophysiological responses in the hippocampus higher than those observed in wild-type animals (Alkondon et al., 2004). On the other hand, very little is known about the possible effects of 5-hydroxyindole and kynurenic acid on non-α7 nAChR function.
To evaluate further the possibility of modulating the function of nAChRs, an experimental approach completely different from those previously employed was adopted in the present investigation. In particular, given the prevalent presynaptic localization of nAChRs, we directly monitored neurotransmitter release from synaptosomes in superfusion (see Raiteri and Raiteri, 2000), which is believed to be an appropriate preparation to study presynaptic events. In addition, the release of [3H]D-aspartate or endogenous glutamate was taken as a functional response linked to activation of α7 nAChRs present on glutamatergic nerve endings (Marchi et al., 2002). Finally, we evaluated and compared non-α7 release-enhancing nAChRs localized on hippocampal noradrenergic or cholinergic terminals and on striatal dopaminergic terminals.
Methods
Animals
Adult Swiss mice (weighing 20–25 g; Charles River, Calco, Italy) were housed at constant temperature (22±1°C) and relative humidity (50%) under a regular light–dark schedule (light 0700–1900 hours). Food and water were freely available. The animals were killed by decapitation and the cerebral cortices were rapidly removed at 0–4°C. The experimental procedures were approved by the Ethics Committee of the Pharmacology and Toxicology Section, Department of Experimental Medicine, in accordance with the European legislation (European Communities Council Directive of 24 November 1986, 86/609/EEC) and with the NIH Guide for the Care and Use of Laboratory Animals.
Brain tissue preparation
Purified cortical, striatal and hippocampal synaptosomes were prepared according to Dunkley et al. (1986), with a few minor modifications. The tissue was homogenized in 10 volumes of 0.32 M sucrose, buffered at pH 7.4 with Tris (final concentration 0.01 M) using a glass Teflon tissue grinder (clearance 0.24 mm). The homogenate was centrifuged at 1000 g for 5 min, to remove nuclei and cellular debris, and the supernatant was gently stratified on a discontinuous Percoll gradient (6, 10 and 20% v/v in Tris-buffered sucrose) and centrifuged at 33 500 g for 5 min. The layer between 10 and 20% Percoll (synaptosomal fraction) was collected and washed by centrifugation. The synaptosomal pellet was then resuspended in physiological medium containing 125 mM NaCl, 3 mM KCl, 1.2 mM MgSO4, 1.2 mM CaCl2, 1 mM NaH2PO4, 22 mM NaHCO3 and 10 mM glucose (pH 7.2–7.4) (gassed with 95% O2 and 5% CO2).
Release experiments
Synaptosomes were incubated at 37°C for 15 min in the absence (experiments of endogenous glutamate release) or presence of [3H]D-aspartate (final concentration 0.08 μM), or [3H]NA (final concentration 0.03 μM) or [3H]choline (final concentration 0.08 μM) or [3H]DA (final concentration 0.05 μM). Selective labelling with [3H]NA was carried out in the presence of 0.1 μM of the serotonin uptake blocker 6-nitroquipazine and of the DA uptake blocker GBR12909. Labelling with [3H]DA was carried out in the presence of 6-nitroquipazine and 0.1 μM of the NA blocker nisoxetine.
At the end of the incubation period, identical portions of the synaptosomal suspension were layered on microporous filters at the bottom of parallel superfusion chambers maintained at 37°C (Raiteri and Raiteri, 2000). Superfusion (0.5 ml min−1) was started with standard physiological medium (see above) gassed with 95% O2 and 5% CO2, at 37°C. Starting from t=37 min to t=41 min of superfusion, six consecutive 1-min fractions were collected. Synaptosomes were exposed to agonists at t=38 min, till the end of superfusion. Antagonists and 5-hydroxyindole were added 8 min before agonists. Samples were collected and the superfused synaptosomes were then counted for radioactivity. Tritiated transmitters were determined in each fraction collected and in the superfusion filters by liquid scintillation counting. The amount of tritium released into each superfusate fraction was expressed as a percentage of the total tissue content at the start of the fraction collected. The evoked overflow was calculated by subtracting the corresponding basal release from each fraction. Appropriate controls with antagonists were always run in parallel. The concentration–response curve was fitted to the experimental data using the following four parameters logistic equation provided by the software Sigma Plot version 8.0 (Jandel Corporation, UK):
where a is the minimum and b the maximum value of the data; c is the EC50 and d is the slope of the curve. The concentration–inhibition curve was fitted to the experimental data using the following equation:
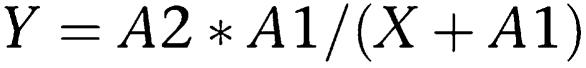 |
where X is the inhibitor dose; A1 is the IC50 and A2 is Y (X=0).
When release was evoked with high K+, the following consecutive samples were collected: basal release (B1; 3 min); K+-evoked release (S; 6 min); after-depolarization basal release (B2; 3 min). Synaptosomes were exposed to the depolarizing stimulus (medium containing 12 mM KCl, with or without agonists) for 90 s, starting at the end of the first fraction collected (B1). Antagonists and 5-hydroxyindole were added 8 min in advance and were also present in the depolarizing stimulus. The K+-evoked overflow was estimated by subtracting the basal release (B1+B2) from the K+-evoked release (S).
Endogenous glutamate determination
Endogenous glutamate was determined by HPLC, with a method routinely used in our laboratory, after precolumn derivatization with o-phthalaldehyde and separation on a C18 reverse-phase chromatographic column (10 × 4.6 mm, 3 μm; 30°C; Chrompack, Middelburg, The Netherlands) coupled with fluorimetric detection (excitation wavelength 350 nm, emission wavelength 450 nm). Buffers and the gradient programme were as follows: solvent A, 0.1 M sodium acetate (pH 5.8)/methanol, 80:20; solvent B, 0.1 M sodium acetate (pH 5.8)/methanol, 20:80; solvent C, 0.1 M sodium acetate (pH 6.0)/methanol, 80:20; the gradient programme was 100% C for 4 min from the initiation of the programme; linear change to 90% A and 10% B from min 4 to min 5; isocratic flow (90% A and 10% B) from min 5 to min 7; linear change to 78% A and 22% B from min 7 to min 9; isocratic flow (78% A and 22% B) from min 9 to min 15; linear change to 66% A 8 and 34% B from min 15 to min 18; linear change to 42% A and 58% B from min 18 to min 19; linear change to 100% B from min 19 to min 20; isocratic flow (100% B) from min 20 to min 22; linear change to 100% C from min 22 to min 25; the total run time was 25 min; flow rate 0.9 ml min−1. Homoserine was used as an internal standard. The endogenous glutamate release was expressed as picomoles per milligram of synaptosomal protein. The evoked overflow was calculated by subtracting the corresponding basal release from each fraction.
Statistical analyses
To analyse the significance between mean values, two-way ANOVA followed by the Newman–Keuls or Dunnett multiple comparison analyses were applied. Direct comparison analysis was performed with the two-tailed Student's t-test. P<0.01 and P<0.05 were considered statistically significant.
Chemicals
1-[7,8-3H]NA (specific activity 35 Ci mmol−1), [3H]D-aspartate (specific activity 16.3 Ci mmol−1), [3H]choline (specific activity 69 Ci mmol−1) and [7,8-3H]DA (specific activity 42 Ci mmol−1) were purchased from Amersham Radiochemical Center (Amersham, UK). 5-hydroxyindole, kynurenic acid, nisoxetine, (−)-nicotine bitartrate, α-bungarotoxin and methyllycaconitine citrate were obtained from Sigma Chemical Co. (St Louis, MO, USA); Percoll from Pharmacia (Uppsala, Sweden). (+)-epibatidine was purchased from RBI (Natick, MA, USA), 6-nitroquipazine maleate (Duphar, Amsterdam, The Netherlands), GBR 12909 (Gist-Brocades, Delft, The Netherlands).
Results
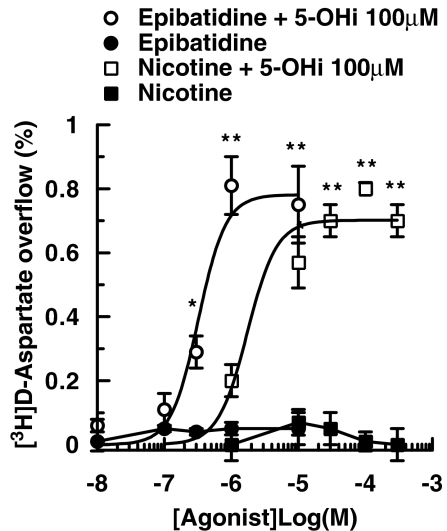
As shown in Figure 1, varying concentrations of epibatidine and nicotine added alone to the superfusion medium were unable to enhance the basal release of tritium from synaptosomes of mouse neocortex, prelabelled with [3H]D-aspartate. The two nAChR agonists exhibited concentration-dependent release of [3H]D-aspartate when 5-hydroxyindole (100 μM), only weakly active on its own (see below), was added to the superfusion solution 8 min before agonists (Figure 1). The EC50 values in the presence of 5-hydroxyindole were 0.32 μM for epibatidine and 3.12 μM for nicotine.
Figure 1.
Effects of 5-hydroxyindole (5-OHi) on the nAChR agonist-mediated overflow of [3H]D-aspartate ([3H]D-ASP) from mouse cortical synaptosomes. 5-OHi was added 8 min before agonists. Data are mean ±s.e.mean of 4–9 experiments run in triplicate (three superfusion chambers for each experimental condition). *P<0.05; **P<0.01 versus control (two-tailed student's t-test).
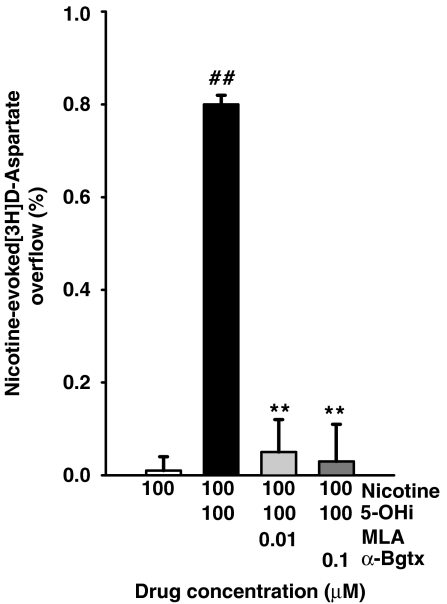
Release of aspartate induced by the combination of 100 μM nicotine plus 100 μM 5-hydroxyindole was abolished by methyllycaconitine (10 nM) or α-bungarotoxin (100 nM), two antagonists with selectivity for the α7 nAChR subtype (Figure 2).
Figure 2.
Antagonism by methyllycaconitine (MLA) and α-bungarotoxin (α-Bgtx) of the [3H]D-aspartate overflow evoked by nicotine plus 5-hydroxyindole (5-OHi) from mouse cortical synaptosomes. Data are means ±s.e.mean of 4–9 experiments run in triplicate. **P<0.01 versus nicotine plus 5-OHi; ##P<0.01 versus nicotine (two-tailed student's t-test).
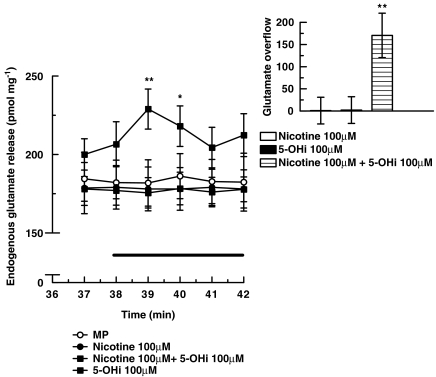
Neurotransmitter release from glutamatergic neurons is often studied by monitoring the efflux of previously taken up [3H]D-aspartate, a convenient approach because D-aspartate is a substrate for glutamate transporters and is not metabolized, but release of this amino acid may differ importantly from that of the endogenously synthesized transmitter (Poli et al., 1985). For this reason, the release of endogenous glutamate was monitored during superfusion of mouse cortical synaptosomes in the presence of nicotine, with or without 5-hydroxyindole. As illustrated in Figure 3, nicotine (100 μM) or 5-hydroxyindole (100 μM) did not evoke, on their own, a significant increase in the release of endogenous glutamate but, when present together in the superfusion medium, they were able to stimulate release. The inset of Figure 3 shows the overflow (total minus basal release) of endogenous glutamate provoked by 100 μM nicotine in the presence of 100 μM 5-hydroxyindole. Because release of endogenous glutamate and of exogenous [3H]D-aspartate responded similarly to the combination of nicotine and 5-hydroxyindole, we subsequently used release of [3H]D-aspartate as an adequate indicator of the release of the endogenous transmitter, glutamate, in our preparations.
Figure 3.
Effects of nicotine and 5-hydroxyindole (5-OHi), alone or in combination, on the release of endogenous glutamate from mouse cortical synaptosomes. 5-Hydroxyindole was added 8 min before nicotine. Data are mean ±s.e.mean of 5–9 experiments run in triplicate. *P<0.05; **P<0.01 versus nicotine (two-tailed student's t-test). Inset: effect of nicotine and 5-hydroxyindole alone or in combination on glutamate overflow (total minus basal release). *P<0.05; **P<0.01 versus nicotine (two-tailed student's t-test).
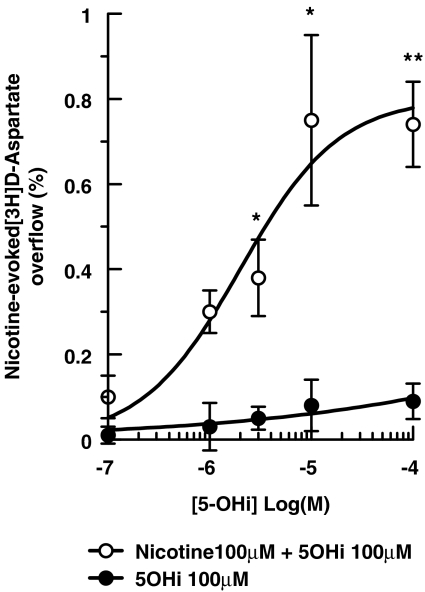
We next assessed modulation of nAChRs by 5-hydroxyindole by measuring [3H]D-aspartate release with a fixed concentration of nicotine (100 μM) and varying concentrations of 5-hydroxyindole (0.1–100 μM), added 8 min before the nicotine. As shown in Figure 4, although 5-hydroxyindole was unable to elicit release on its own, in combination with nicotine it permitted significant activation of α7 nAChRs at a concentration of 3 μM, with a maximal effect at about 100 μM.
Figure 4.
Concentration-response relationship of the effects of 5-hydroxyindole (5-OHi) on the nicotine-evoked [3H]D-aspartate overflow from mouse cortical synaptosomes. Data are means ±s.e.mean of three experiments run in triplicate. *P<0.05; **P<0.01 versus control (two-tailed student's t-test).
The presynaptic α7 nAChRs on glutamatergic terminals of rat striatum and human neocortex respond to nAChR agonists when the membranes on which they are localized are moderately depolarized (i.e. by 12 mM KCl), but not under basal conditions (Marchi et al., 2002). As detailed in Table 1, in rat striatum and human neocortex, epibatidine (1 μM) significantly enhanced the [3H]D-aspartate release evoked by 12 mM K+. However, the overflow of [3H]D-aspartate could not be further increased when 5-hydroxyindole was added together with high K+ and epibatidine, nor was the K+-evoked overflow of [3H]D-aspartate modified by 5-hydroxyindole (100 μM), given alone.
Table 1.
Effects of epibatidine on the release of [3H]D-aspartate in the presence of KCl, 5-hydroxyindole (5-OHi) or both
| Drugs | [3H]D-aspartate (% of overflow) |
|---|---|
| KCl 12 mM | 2.30±0.06 |
| Epibatidine 1 μM+KCl | 2.71±0.09a |
| Epibatidine+KCl+5-OHi 100 μM | 2.62±0.10a |
| KCl+5-OHi | 2.29±0.04 |
Data are means±s.e.mean of three experiments run in quadruplicate (four superfusion chambers for each condition).
Significantly different from KCl control, P<0.05 one-way ANOVA, with post hoc Dunnett test.
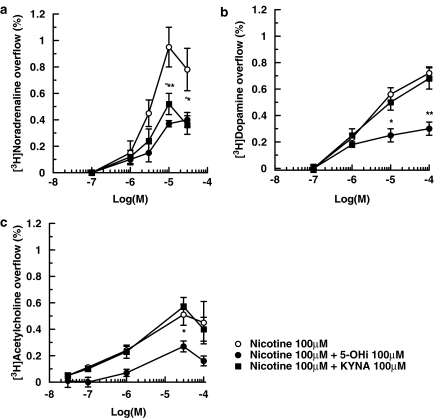
Subsequently, we investigated the selectivity of the 5-hydroxyindole effect on nAChR subtypes by measuring transmitter release from noradrenergic, dopaminergic and cholinergic terminals. The nAChRs on these terminals behave pharmacologically as non-α7 nAChRs (Wonnacott, 1997; Champtiaux et al., 2003; Salminen et al., 2004). Mouse hippocampal synaptosomes were prelabelled with [3H]NA or [3H]choline to study the release of NA and ACh; striatal synaptosomes were prelabelled with [3H]DA to study DA release. Figure 5a–c shows that nicotine increased, in a concentration-dependent manner, the release of [3H]NA, [3H]DA and [3H]ACh from mouse brain tissues. Interestingly, 5-hydroxyindole inhibited the release of the three [3H] transmitters when added over the same concentration range as used in our experiments with α7 nAChRs on glutamatergic terminals (see above).
Figure 5.
Effects of 5-hydroxyindole (5-OHi) or kynurenic acid (KYNA) on the nicotine-evoked overflow of [3H]NA from hippocampal synaptosomes (a) of [3H]DA from striatal synaptosomes (b) and of [3H]ACh from hippocampal synaptosomes (c). 5-Hydroxyindole or kynurenic acid were added 8 min before nicotine. Data are means ±s.e.mean of 4–9 experiments run in triplicate. *P<0.05; **P<0.01 (5-OHi versus nicotine); °P<0.05 (KYNA versus nicotine) (two-tailed student's t-test).
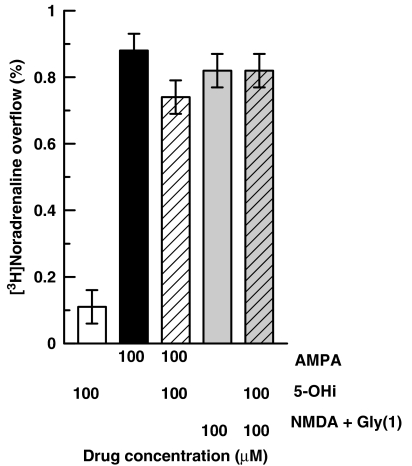
Presynaptic ionotropic glutamate receptors of the NMDA and AMPA type exist on noradrenergic nerve endings of the rat hippocampus where they mediate NA release (Pittaluga and Raiteri, 1992; Risso et al., 2004). As shown in Figure 6, AMPA (100 μM) or NMDA (100 μM) plus glycine (1 μM) elicited release of [3H]NA from mouse hippocampal synaptosomes. However, at a concentration of 100 μM, 5-hydroxyindole affected neither basal release (given alone) nor that stimulated by AMPA or NMDA.
Figure 6.
Effects of 5-hydroxyindole (5-OHi) on the overflow of [3H]NA evoked by AMPA or by NMDA plus glycine from mouse hippocampal synaptosomes. 5-Hydroxyindole, NMDA and AMPA were used at 100 μM; glycine at 1 μM. Data are means ±s.e.mean of 4–9 experiments run in triplicate.
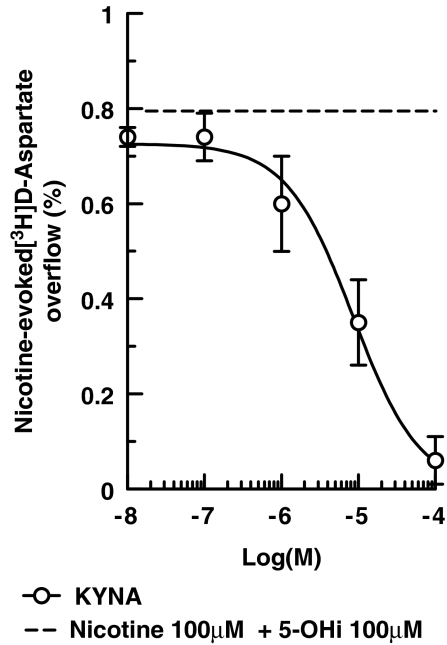
Another product of tryptophan metabolism, kynurenic acid, also appears to be a blocker of α7 nAChRs (Hilmas et al., 2001; Alkondon et al., 2004; Rassoulpour et al., 2005). As illustrated in Figure 7, kynurenic acid concentration-dependently inhibited the release of [3H]D-aspartate evoked by nicotine (100 μM) plus 5-hydroxyindole (100 μM) from mouse neocortical synaptosomes; the calculated IC50 was 8.25±0.97 μM.
Figure 7.
Antagonism by kynurenic acid of the overflow of [3H]D-aspartate evoked by nicotine plus 5-hydroxyindole from mouse cortical synaptosomes. Kynurenic acid and 5-hydroxyindole were added 8 min before nicotine. Data are means ±s.e.mean of five experiments run in triplicate.
The effects of kynurenic acid on the release of [3H]NA, [3H]DA and [3H]ACh evoked by nicotine (0.1–100 μM; in the absence of 5-hydroxyindole) are presented in Figure 5. When kynurenic acid was added at 100 μM (the concentration that abolished the release of [3H]D-aspartate evoked by 100 μM nicotine plus 100 μM 5-hydroxyindole; see Figure 7), release of [3H]NA evoked by 10 or 30 μM nicotine was significantly depressed (Figure 7a). At a lower concentration (10 μM), kynurenic acid did not affect nicotine-evoked release of [3H]NA (data not shown). Figure 5 also illustrates that 100 μM kynurenic acid did not affect the release of [3H]DA (Figure 5b) or [3H]ACh (Figure 5c) elicited by varying concentrations of nicotine.
Discussion
Agonists at the nAChR were unable to elicit release of glutamate when added to rat striatal and human neocortical synaptosomes (Marchi et al., 2002) or to rat hippocampal and cortical synaptosomes (Grilli et al., 2005; Patti et al., 2006) when the membrane potential was at the resting level. The receptors involved, nAChRs of the α7 subtype, seem to require moderate membrane depolarization (as induced by solutions containing 12 mM K+) to become functional. These observations, together with the present results with mouse neocortical nerve terminals, suggest that the lack of response to agonists of the presynaptic α7 nAChRs localized on ‘resting' glutamatergic axon terminals could be a characteristic of these nerve endings, with the possible exception of those innervating the rat frontal cortex (Rousseau et al., 2005). In contrast, the non-α7 nAChRs sited on noradrenergic, dopaminergic and cholinergic terminals do respond to nicotinic agonists under basal conditions (cf. Figures 1 and 5; Wonnacott, 1997 for a review).
One major finding of the present work is that, when 5-hydroxyindole was present in the superfusion solution, the nAChR agonists tested exhibited concentration-dependent release of transmitter, both in terms of [3H]D-aspartate (representing the newly taken up transmitter) and of endogenous glutamate, without K+ depolarization. At mossy fibre–granule cell synapses, application of 1 mM ACh alone was found to induce glutamate-evoked excitatory postsynaptic currents and coapplication of 1 mM 5-hydroxyindole with 1 mM ACh further increased the frequency of these currents. The ACh-induced current, as well as that caused by 5-hydroxyindole plus ACh, was mediated by α7 nAChRs (Zwart et al., 2002). Why these α7 nAChRs appear to respond to the nicotinic agonist alone is unclear, especially when the same type of receptor in our system did not respond. One reason may be that Zwart et al. (2002) used cerebellar slices from immature rats, whereas our experiments were performed with nerve terminals isolated from mature mouse cerebrocortex. Regardless of the explanation, Zwart et al. (2002) report their finding as a potentiation by 5-hydroxyindole of a response mediated by α7 nAChRs, whereas our findings are more precisely described as a permissive role of 5-hydroxyindole on the activation of presynaptic α7 nAChRs. Interestingly, very low concentrations of 5-hydroxyindole were sufficient to permit α7 nAChR activation because significant effects on glutamate release could be observed when 5-hydroxyindole was present at 3 μM (see Figure 4), that is, at a concentration of 2–3 orders of magnitude lower than those (1 mM or higher) generally employed in electrophysiological studies.
These results indicate that activation of presynaptic α7 nAChRs on glutamatergic terminals does not necessarily require depolarization of the terminal membrane. In fact, 5-hydroxyindole exhibited no releasing effect on its own, differing from the mild depolarizing stimulus (12 mM KCl) previously found to permit α7 nAChRs activation and elicit glutamate release (Marchi et al., 2002). The permissive effect of high K+ on the activation of α7 nAChRs by epibatidine was not further increased by 5-hydroxyindole. This apparent lack of additivity can have various explanations including the possibility that high K+ or 5-hydroxyindole permits maximal receptor activation through different mechanisms, which deserve further investigation. If endogenous 5-hydroxyindole is insufficiently or excessively produced, its ability to permit activation of α7 nAChRs mediating glutamate release may have pathophysiological relevance.
5-Hydroxyindole, a serotonin analogue, also potentiated the function of the ionotropic 5-HT3 receptors (Kooyman et al., 1993; Van Hooft et al., 1997). However, involvement of 5-HT3 receptors in the present study with 5-hydroxyindole should be excluded for at least two reasons. First, the release evoked by 5-hydroxyindole plus nicotine was abolished by methyllycaconitine or α-bungarotoxin, two antagonists, which, at the concentrations used (10 and 100 nM, respectively), should block selectively α7 nAChRs. Second, the presence of endogenously released 5-HT at relevant concentrations is unlikely because, in our superfusion technique, any compound released is removed by the medium superfusing a monolayer of synaptosomes before it can activate presynaptic targets on the releasing terminals or on neighbouring structures (see Raiteri and Raiteri, 2000 for technical details).
Surprisingly, the well-known enhancing effects of nicotine on the basal release of NA, DA or ACh were strongly inhibited by 5-hydroxyindole in the same concentrations that permitted maximal effects of nicotine on the release of glutamate. This inhibition occurred at various concentrations of nicotine. The maximal effects of nicotine on the release of NA, DA or ACh were inhibited by at least 50% in the presence of 5-hydroxyindole, suggesting that this modulator decreases the effect of nicotine by acting as a non-competitive antagonist and decreasing receptor availability. These results imply a striking diversity in the response to 5-hydroxyindole of α7 versus non-α7 nAChRs. Previous electrophysiological results show a different outcome: coapplication of 5-hydroxyindole and ACh exhibited no significant changes of somatic currents mediated by non-α7 nAChRs, mainly of the α4β2 subtype (Zwart et al., 2002), whereas whole-cell currents mediated by nicotine through α4β2 nAChRs stably expressed in HEK293 cells were inhibited by 1 mM 5-hydroxyindole (Fucile et al., 2004).
The mechanism by which 5-hydroxyindole permits α7 nAChRs to exhibit function remains speculative. Although it is reasonable to assume that K+ depolarization provides Ca2+ through voltage-sensitive channels and Ca2+, added to the ions entering through the α7 channel (per se insufficient to elicit release) can trigger glutamate exocytosis, it seems very unlikely that 5-hydroxyindole causes depolarization on its own. According to Zwart et al. (2002), 5-hydroxyindole would increase the affinity as well as the efficacy of ACh at α7 nAChRs, as proposed for the effect of 5-hydroxyindole on 5-HT3 receptors (Van Hooft et al., 1997). Despite the uncertainty of the mechanism by which this process takes place, 5-hydroxyindole appears to represent a tool able to affect, positively, the function of presynaptic α7 nAChRs, whereas negatively affecting that of various non-α7 receptors and could therefore be useful in characterizing the function of nAChR subtypes.
The α7 subunit is present at a particularly high density in cortical areas and the hippocampus (Dominguez del Toro et al., 1994) and seems to participate in a range of important processes including cognitive function (Wonnacott, 1997; Levin and Rezvani, 2000; Picciotto and Zoli, 2002). Therefore, drugs that can facilitate the function of α7 nAChRs could help in the treatment of neuropsychiatric diseases like schizophrenia and neurodegenerative disorders like Alzheimer's disease in which the populations of α7-binding sites are decreased in selected brain regions (Freedman et al., 2000; Court et al., 2001).
The astrocyte-derived factor, kynurenic acid, is a product of tryptophan metabolism endowed with neuroprotective and neuroinhibitory properties. These have been generally attributed to its ability to antagonize competitively, at high micromolar concentrations, the glycine site of glutamate NMDA receptors (Stone, 1993; Pellicciari et al., 1994). On the other hand, the relatively low physiological brain levels of kynurenic acid exhibit clear changes in several diseases: decreases in patients with end-stage Parkinson's disease (Ogawa et al., 1992) and Huntington's disease (Beal et al., 1992) were observed, whereas increases of kynurenic acid brain levels may occur in patients with Alzheimer's disease (Baran et al., 1999) and schizophrenia (Schwarcz et al., 2001).
Exposure of cultured hippocampal neurons to kynurenic acid inhibited activation of somatodendritic α7 nAChRs by ACh (IC50≈7 μM) in a non-competitive manner and more potently than glutamate NMDA receptor activation by NMDA plus glycine (Hilmas et al., 2001). Here, we showed that kynurenic acid can inhibit the activation of presynaptic α7 nAChRs by nicotine plus 5-hydroxyindole with a potency (IC50=8.52 μM) very close to that reported by Hilmas et al. (2001). Significant inhibition could be seen at kynurenic acid concentration of 1 μM (see Figure 7), compatible with the levels found in human brain under pathological conditions (Moroni et al., 1988; Turski et al., 1988; Schwarcz and Pellicciari, 2002). As kynurenic acid inhibited the non-α7 nAChR-mediated release of NA only at high concentrations (>10 μM which are of biochemical, but not pathophysiological relevance), kynurenic acid may be considered as a reasonably selective blocker of α7 nAChR function, as previously proposed (Hilmas et al., 2001; Alkondon et al., 2004; Rassoulpour et al., 2005).
In conclusion, it seems that the two tryptophan metabolites, 5-hydroxyindole and kynurenic acid, can produce opposite effects on the function of release-enhancing presynaptic α7 nAChRs situated on glutamatergic axon terminals. Further studies are required to explore the detailed mechanism of these functional modulations, as a prerequisite to the development of novel drugs able to facilitate α7 nAChR activity or/and to weaken the impact of pathologically elevated kynurenic acid levels on these receptors.
When this work was under review, Mok and Kew (2006) published a report showing that, in electrophysiological experiments, 5-hydroxyindole facilitated GABAergic transmission via excitation of postsynaptic α7 nAChRs sited on GABAergic interneurons of CA1, an effect requiring the presence of the endogenous agonist ACh in the extracellular environment of the receptor. In spite of the marked differences between the experimental conditions used by Mok and Kew (2006) and in the present study, there is agreement on the idea that 5-hydroxyindole is a positive modulator at α7 nAChRs, located in different parts of the rodent brain.
Acknowledgments
This work was supported by grants from the Italian MIUR. We thank Mrs Maura Agate for her collaboration in preparing the paper and Silvia E Smith for reading the paper.
Abbreviations
- DA
dopamine
- NA
noradrenaline
- nAChRs
nicotinic acetylcholine receptors
Conflict of interest
The authors state no conflict of interest.
References
- Alkondon M, Pereira EFR, Yu P, Arruda EZ, Almeida LEF, Guidetti P, et al. Targeted deletion of the kynurenine aminotransferase II gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via α7 nicotinic receptors in the hippocampus. J Neurosci. 2004;24:4635–4648. doi: 10.1523/JNEUROSCI.5631-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran H, Jellinger K, Deecke L. Kynurenine metabolism in Alzheimer's disease. J Neural Transm. 1999;106:165–181. doi: 10.1007/s007020050149. [DOI] [PubMed] [Google Scholar]
- Beal MF, Matson WR, Storey E, Milbury P, Ryan EA, Ogawa T, et al. Kynurenic acid concentrations are reduced in Huntington's disease cerebral cortex. J Neurol Sci. 1992;108:80–87. doi: 10.1016/0022-510x(92)90191-m. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court J, Martin-Ruiz C, Piggott M, Spurden D, Griffiths M, Perry E. Nicotinic receptor abnormalities in Alzheimer's disease. Biol Psychiatry. 2001;49:175–184. doi: 10.1016/s0006-3223(00)01116-1. [DOI] [PubMed] [Google Scholar]
- Dominguez del Toro E, Juiz JM, Peng X, Lindstrom J, Criado M. Immunocytochemical localization of the alpha 7 subunit of the nicotinic acetylcholine receptor in the rat central nervous system. J Comp Neurol. 1994;349:325–342. doi: 10.1002/cne.903490302. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Heath JW, Kidd GJ, Rostas JAP. A rapid method for isolation of synaptosomes on percoll gradients. Brain Res. 1986;372:115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adams CE, Leonard S. The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanat. 2000;20:299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- Fucile S, Renzi M, Lauro C, Limatola C, Ciotti T, Eusebi F. Nicotinic cholinergic stimulation promotes survival and reduces motility of cultured rat cerebellar granule cells. Neuroscience. 2004;127:53–61. doi: 10.1016/j.neuroscience.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Grilli M, Parodi M, Raiteri M, Marchi M. Chronic nicotine differentially affects the function of nicotinic receptor subtypes regulating neurotransmitter release. J Neurochem. 2005;93:1353–1360. doi: 10.1111/j.1471-4159.2005.03126.x. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EFR, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooyman AR, van Hooft JA, Vijverberg HPM. 5-Hydroxyindole slows desensitization of the 5-HT3 receptor-mediated ion current in N1E-115 neuroblastoma cells. Br J Pharmacol. 1993;108:287–289. doi: 10.1111/j.1476-5381.1993.tb12795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Development of nicotinic drug therapy for cognitive disorders. Eur J Pharmacol. 2000;393:141–146. doi: 10.1016/s0014-2999(99)00885-7. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Carpenedo R, Moroni F. 5-hydroxyindole causes convulsions and increases transmitter release in the CA1 region of the rat hippocampus. Br J Pharmacol. 2003;138:245–253. doi: 10.1038/sj.bjp.0705007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating α7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem. 2002;80:1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- Mok MHS, Kew NCJ. Excitation of hippocampal interneurons via modulation of endogenous agonist activity at the 7 nicotinic ACh receptor. J Physiol. 2006;574:699–710. doi: 10.1113/jphysiol.2006.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F, Russi P, Lombardi G, Beni M, Carlà V. Presence of kynurenic acid in the mammalian brain. J Neurochem. 1988;51:177–180. doi: 10.1111/j.1471-4159.1988.tb04852.x. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Matson WR, Beal MF, Myers RH, Bird ED, Milbury P, et al. Kynurenine pathway abnormalities in Parkinson's disease. Neurology. 1992;42:1702–1706. doi: 10.1212/wnl.42.9.1702. [DOI] [PubMed] [Google Scholar]
- Patti L, Raiteri L, Grilli M, Parodi M, Raiteri M, Marchi M. P2X7 receptors exert a permissive role on the activation of release-enhancing presynaptic α7 nicotinic receptors co-existing on rat neocortex glutamatergic terminals. Neuropharmacology. 2006;50:705–713. doi: 10.1016/j.neuropharm.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Natalini B, Costantino G, Mahmoud MR, Mattoli L, Sadeghpour BM, et al. Modulation of the kynurenine pathway in search for new neuroprotective agents. Synthesis and preliminary evaluation of (m-nitrobenzoyl)alanine, a potent inhibitor of kynurenine-3-hydroxylase. J Med Chem. 1994;37:647–655. doi: 10.1021/jm00031a015. [DOI] [PubMed] [Google Scholar]
- Pereira EF, Hilmas C, Santos MD, Alkondon M, Maelicke A, Albuquerque EX. Unconventional ligands and modulators of nicotinic receptors. J Neurobiol. 2002;53:479–500. doi: 10.1002/neu.10146. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Nicotinic receptors in aging and dementia. J Neurobiol. 2002;53:641–655. doi: 10.1002/neu.10102. [DOI] [PubMed] [Google Scholar]
- Pittaluga A, Raiteri M. N-methyl-D-aspartic acid (NMDA) and non-NMDA receptors regulating hippocampal norepinephrine release. III. Changes in the NMDA receptor complex induced by their functional cooperation. J Pharmacol Exp Ther. 1992;263:327–333. [PubMed] [Google Scholar]
- Poli A, Contestabile A, Migani P, Rossi L, Rondelli C, Virgili M, et al. Kainic acid differentially affects the synaptosomal release of endogenous and exogenous amino acidic neurotransmitters. J Neurochem. 1985;45:1677–1686. doi: 10.1111/j.1471-4159.1985.tb10522.x. [DOI] [PubMed] [Google Scholar]
- Raiteri L, Raiteri M. Synaptosomes still viable after 25 years of superfusion. Neurochem Res. 2000;25:1265–1274. doi: 10.1023/a:1007648229795. [DOI] [PubMed] [Google Scholar]
- Rassoulpour A, Wu H-Q, Ferré S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- Risso F, Grilli M, Parodi M, Bado M, Raiteri M, Marchi M. Nicotine exerts a permissive role on NMDA receptor function in hippocampal noradrenergic terminals. Neuropharmacology. 2004;47:65–71. doi: 10.1016/j.neuropharm.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Rousseau SJ, Jones IW, Pullar IA, Wonnacott S. Presynaptic α7 and non-α7 nicotinic acetylcholine receptors modulate [3H]D-aspartate release from rat frontal cortex in vitro. Neuropharmacology. 2005;49:59–72. doi: 10.1016/j.neuropharm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–379. [PubMed] [Google Scholar]
- Turski WA, Nakamura M, Todd WP, Carpenter BK, Whetsell WO, Jr, Schwarcz R. Identification and quantification of kynurenic acid in human brain tissue. Brain Res. 1988;454:164–169. doi: 10.1016/0006-8993(88)90815-3. [DOI] [PubMed] [Google Scholar]
- Van Hooft JA, Van der Haar E, Vijverberg HP. Allosteric potentiation of the 5-HT3 receptor-mediated ion current in N1E-115 neuroblastoma cells by 5-hydrxyindole and analogues. Neuropharmacology. 1997;36:649–653. doi: 10.1016/s0028-3908(97)00045-2. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Zwart R, De Filippi G, Broad LM, McPhie GI, Pearson KH, Baldwinson T, et al. 5-Hydroxyindole potentiates human alpha 7 nicotinic receptor-mediated responses and enhances acetylcholine-induced glutamate release in cerebellar slices. Neuropharmacology. 2002;43:374–384. doi: 10.1016/s0028-3908(02)00094-1. [DOI] [PubMed] [Google Scholar]