Abstract
Background and Purpose:
Protease-activated receptor-2 (PAR-2) is expressed on lymphocytes and endothelial cells, and plays a significant role in inflammatory reactions. Since leukocyte-endothelial cell interaction and reactive oxygen species (ROS) generation are hallmarks of the development of inflammation, the effects of PAR-2 activation by trypsin on lymphocyte adhesion and ROS generation was examined utilising PAR-2 wild type and knockout (PAR-2−/−) mice.
Experimental Approach:
Lymphocyte adhesion to the luminal surface of mouse isolated aortae was measured using 51Cr-labelled leukocytes and ROS generation from isolated lymphocytes was quantified using chemiluminescence.
Key results:
Trypsin induced adhesion of lymphocytes when added exogenously to the endothelial surface of the aorta for 30 min. Similarly, increased lymphocyte adhesion was also observed when mice were injected with trypsin intravenously 24 h prior to the adhesion assay, an effect which was partly ICAM-1 mediated. Trypsin also increased ROS generation from isolated mouse lymphocytes in a dose-dependent manner. The increase in lymphocyte adhesion and ROS production in response to trypsin were abolished in PAR-2−/− mice indicating a PAR-2 dependent mechanism. Superoxide dismutase had a greater inhibitory effect in PAR-2−/− mice compared to wild type mice when lymphocytes were stimulated with PMA but not trypsin.
Conclusions and Implications:
The present study indicates that activation of PAR-2 may be an important factor in modulating lymphocyte adhesion and ROS generation. The results have implications for developing anti-inflammatory strategies.
Keywords: cell trafficking, inflammation, knockout mice, T cells, B cells, protease-activated receptor-2, reactive oxygen species
Introduction
Protease-activated receptors (PARs) belong to a novel emerging family of cell surface, seven-transmembrane domain, G-protein coupled receptors that are irreversibly activated by serine proteases. Activation of the receptor is initiated by proteolytic unmasking of the N-terminal sequence of the receptor to expose a ‘tethered ligand' that in turn binds intramolecularly within the extracellular loop-2 of the receptor. As a result, the receptor in the active conformation interacts with the heterodimeric plasma membrane G-proteins to transduce the intracellular signal (Vu et al., 1991; Coughlin, 2000). PAR-2 was discovered initially by reduced stringency cloning from a mouse genomic library. Trypsin, being one of the endogenous activators for PAR-2, cleaves the N-terminal extracellular domain at SKGR34/S35LIG to generate a ‘tethered ligand' protein sequence, SLIGRL, to activate the receptor (Nystedt et al., 1994).
Functional PAR-2 is widely expressed on various immune cells such as neutrophils (Howells et al., 1997), lymphocytes (Mari et al., 1996), eosinophils (Miike et al., 2001; Bolton et al., 2003) and monocytes (Colognato et al., 2003), as well as the vascular endothelium and smooth muscle cells (D'Andrea et al., 1998). Early studies showed that PAR-2 expression was selectively upregulated by inflammatory mediators such as tumour necrosis factor α (TNFα), interleukin-1α and bacterial lipopolysaccharide (Nystedt et al., 1996). In addition, activation of PAR-2 results in various vascular and cellular events associated with the cardinal signs of inflammation such as vasorelaxation (Damiano et al., 1999), increased vascular permeability (Kawabata et al., 1998), induced leucocyte adhesion and infiltration (Vergnolle et al., 1999; Linder et al., 2000) and pain (Dai et al., 2004).
Lymphocytes, mainly cytotoxic T cells and natural killer T cells, play an important role in the early inflammatory response as well as the late adaptive immune response of various vascular inflammatory diseases such as atherosclerosis (Hallenbeck et al., 2005) and ischaemia/reperfusion injury (Varda-Bloom et al., 2000; Ysebaert et al., 2004). Lymphocyte–endothelial adhesive interactions are a fundamental feature in the cascade of events that leads to inflammatory responses. This process requires the expression of, and interaction between, adhesion molecules on both lymphocytes and vascular endothelium and is influenced by various factors including chemoattractants, cytokines, chemokines, nitric oxide, leucotrienes and others. Activated lymphocytes can further promote leucocyte infiltration, activate other leucocyte subtypes and endothelial cells, and induce cellular necrosis and apoptosis directly through the release of various pro-inflammatory mediators such as reactive oxygen species (ROS), cytokines, proteases, complement fragments and others (Carlos and Harlan, 1994; Shresta et al., 1998; Hallenbeck et al., 2005).
Although a growing body of literature implicates PAR-2 in inflammation, there is little information on the function of lymphocytes in adhesion and ROS generation and the effect of PAR-2 activation on these processes.
Materials and methods
Animal sources
Experiments using animals were carried out in accordance with the United Kingdom Home Office Guide on the Operation of Animals (Scientific Procedures) Act 1986 under project licence number 60/2095. Animals were allowed free access to food and water and maintained on a 12 h light and dark cycle. Homozygous PAR-2-deficient (PAR-2−/−) mice and wild-type littermates (PAR-2+/+) were generated as described previously (Ferrell et al., 2003).
Lymphocyte isolation and characterization
Male/female mice (7–10 weeks, weighing 14–34 g) were killed by carbon dioxide asphyxiation. Spleen lymphocytes were isolated based on a published method (Klein et al., 2006). Briefly, the spleen was removed and disrupted by rubbing over a Nitex mesh (200 μm2 pore size) in 3 ml of medium (Rosewell Park Memorial Institute (RPMI)-1640 (Dutch Modification) containing 10% v/v Australia foetal bovine serum). The cell suspension was filtered through a fresh piece of Nitex mesh and centrifuged at 67 g for 10 min. The resultant cell pellet was hypotonically lysed with distilled water for 30 s to eliminate contaminating erythrocytes before an equal volume of 1.8% sodium chloride was added to restore isotonicity. The cell suspension was filtered through a fresh piece of Nitex mesh and centrifuged at 67 g for 10 min to yield a lymphocyte pellet, which was then resuspended in medium. Cell viability was assessed by Trypan blue exclusion and the concentration of lymphocytes was quantified using a haemocytometer slide and the final concentration was adjusted to 106 cells ml−1 with medium.
Spleen cell isolates were characterized in three separate mice using flow cytometry (Kerkvliet and Brauner, 1990). Briefly, cell suspensions were incubated with FcR blocking buffer (anti-CD16/32 hybridoma supernatant, 10% mouse serum and 0.1% azide) for 5 min at 4°C to prevent binding of antibody to cells via Fc regions. The cell suspensions were then incubated with a mixture of cell lineage-specific antibodies for 40 min at 4°C. B lymphocytes were identified using fluorescein isothiocyanate conjugated anti-CD45R/B220 (clone RA3-6B2; BD Pharmingen, Oxford, UK), CD4+ T lymphocytes using Peridinin chlorophyll protein-cyanin 5.5 (PerCP) conjugated anti-CD4 (clone GK1.5; BD Pharmingen, Oxford, UK) and myeloid cells using phycoerythrin-conjugated anti-CD11b (clone M1/70; BD Pharmingen, Oxford, UK). The cells were washed in fluorescent-activated cell sorting (FACS) buffer (phosphate-buffered saline (PBS), 2% foetal calf serum and 0.1% azide) before acquisition using a BD FACSCanto flow cytometer with FACSDiVa software (BD, UK). FlowJo software (Tree Star Inc., USA) was used for three colour analysis. To ascertain which cell types were adhering to the artery surface, cells were added to pinned out segments of aorta for 30 min and adherent cells were harvested by addition of ice-cold PBS solution. The solutions of adherent cells were incubated with the same three antibodies and run through the flow cytometer.
Preparation of artery segments
Autologous thoracic aorta, of approximately 7 mm length, was removed and opened longitudinally. Arteries were then pinned, luminal-side up onto Sylgard blocks (Dow Corning, Germany) and placed in a 37°C humidified chamber. To assess the effect of artery preparation and trypsin incubation on endothelial integrity, aortae from three separate mice were treated with either saline or trypsin solution (1000 U ml−1) for 30 min. After washing, segments were incubated briefly with 1% Toluidine Blue, washed and photographed.
Lymphocyte adhesion assay
Adhesion of lymphocytes to the endothelial surface of aortae was measured using (51Cr)-labelled lymphocytes (Kennedy et al., 2000). A 1 ml aliquot of lymphocyte suspension containing 106 cells ml−1 was labelled for 1 h at 37°C with 185 kBq of 51Cr and agitated every 15 min to reduce cell sedimentation. The lymphocytes were washed twice by centrifugation at 5670 g for 5 min and resuspended in medium at 106 cells ml−1. Artery segments were incubated with 5 μl of the labelled lymphocytes for 30 min and then washed with medium before radioactivity was quantified using a gamma counter (Cobra Auto-gamma, Packard Canberra Company, UK). Lymphocyte adhesion was expressed as the percentage of the lymphocytes added that remained adherent using the following calculation:-
 |
where γArtery=counts from the artery segment and γLymphocytes=count from a 5 μl aliquot of labelled lymphocytes.
For the adhesion study, 10 μl of saline (vehicle control), trypsin (1000 U ml−1 (∼5 μM), 30 min), TNFα (from mouse, 10 ng ml−1, 60 min) or phorbol 12-myristate 13-acetate (PMA; 50 ng ml−1, 30 min) was added to the artery segments before the addition of labelled lymphocytes. For the ex vivo study, trypsin (1 U g−1, in a volume of 0.05 ml per 25 g body weight) was administered via the tail vein using a diabetic syringe. Mice were then killed by CO2 asphyxiation 4 or 24 h later and the lymphocytes and arteries were prepared as described above to measure lymphocyte adhesion. Mice receiving saline (0.05 ml per 25 g body weight) 24 h before leucocyte adhesion assay were used as a vehicle control.
Immunocytochemistry
Immunocytochemical techniques were used to identify expression of adhesion molecules on the arteries using antibodies directed against P-selectin and intercellular cell adhesion molecule (ICAM)-1 followed by a streptavidin–biotin-peroxidase method (Kennedy et al., 2000). Following the adhesion assay, arteries were fixed in formalin 10% neutral buffer. The arteries were then processed in an enclosed tissue processor through a series of graded alcohols and histoclear and embedded end-on in paraffin wax, allowing transverse sections to be cut at 4 μm and mounted onto 3-aminopropyltriethoxy silane-coated glass slides. After rehydration by passage through histoclear and graded alcohols to water, endogenous peroxidase was quenched by immersion in 0.3% hydrogen peroxide for 10 min followed by a 5 min wash in PBS (pH 7.6). Tissue antigens were retrieved by immersion for 5 min in a preheated solution containing ethylenediaminetetraacetic acid/Tris base (0.37/0.55 mg ml−1) at 15 lb in−2 using a microwave and a pressure cooker. The tissue was then air cooled for 20 min before a 5 min PBS wash. After 5 min wash in PBS, the tissue was serially incubated with 20% normal rabbit serum in PBS for 20 min. The tissue was then incubated for 1 h with the primary antibody (anti-P-selectin (M-20) or anti-ICAM-1 goat polyclonol antibody at 1:50 dilution), biotinylated rabbit anti-goat secondary antibody (1:400) for 30 min, streptavidin-horse radish peroxide for 30 min, and 0.05% diaminobenzidine in Tris buffer containing 0.8 ml of 30% hydrogen peroxide, with each incubation process separated by a 5 min PBS wash. Subsequently, the tissue was counterstained by sequential immersion in Haematoxylin Gill II formula (5 min), acid alcohol (four dips), Scott's Tap Water Substitute (0.35% NaHCO3+2% MgSO4 in water, 3 min) and 0.5% copper sulphate in PBS (10 min), where each incubation was separated by 1 min wash in running water, and mounted with DPX mountant after tissue dehydration through graded alcohols to histoclear. The intensity of staining for adhesion molecules was assessed semiquantitatively by an experienced consultant pathologist and scored using a scale as follows: 0 for no apparent staining, 0.5 for very mild positivity, 1 for mild positivity, 1.5 for mild-to-moderate positivity, 2 for moderate positivity, 2.5 for moderate to intense positivity, and 3 for intense positivity. Staining was graded by a second, blinded observer to confirm results.
Chemiluminescence
Luminol-enhanced chemiluminescence was employed for measuring ROS generation from isolated lymphocytes and was measured in a chemiluminometer (Lumi-vette, Chrono-log Corp., Havertown, USA) (Demiryurek et al., 1994). 450 μl of lymphocyte suspension (containing 4.5 × 105 cells) was diluted with 450 μl of PBS in a 1 ml cuvette (Labmedics Ltd, Cheshire, UK) containing a stir bar. Following preincubation at 37°C for 5 min, 10 μl of PMA (50–1000 ng ml−1), platelet-activating factor (PAF, 0.01–50 μM), or trypsin (10–1000 U ml−1) was added immediately after the addition of 100 μl of luminol solution (3-aminophthalhydrazide, 0.04% w/v). Cumulative chemiluminescence was measured at 37°C for 15 min after a 2 min delay period (Demiryurek et al., 1994). To investigate the effect of various ROS scavengers on PMA-stimulated lymphocytes, 10 μl of superoxide dismutase (SOD from bovine erythrocyte, 5–50 U ml−1), catalase (from bovine liver, 300–3000 U ml−1), sodium azide (10−7–10−5 M) or a combination of SOD, catalase and sodium azide was added 1 min before the addition of luminol solution and 10 ng ml−1 of PMA. Results are presented as arbitrary units (instrument readout proportional to photons of light detected by the photomultiplier tube) after correction for readings obtained with the vehicle control (saline) and as a percentage of the total chemiluminescence generated in response to 10 ng ml−1 of PMA alone, respectively. The effect of SOD on trypsin (1000 U ml−1)-induced chemiluminescence was also studied and calculated as a percentage of the total chemiluminescence signal generated in response to saline (basal).
Statistical analysis
All results are shown as mean±s.e.m. and n indicates the number of animals. Student's unpaired t-test was employed to determine the significance of changes between PAR-2+/+ and PAR-2−/− mice or between drug treatments. Statistical significance for the chemiluminescence concentration–response curve was determined using two-way analysis of variance (ANOVA). When significant interactions were detected further analyses were undertaken using Student's unpaired t-test for differences between strains at selected concentrations. One-way ANOVA followed by a Dunnett's multiple comparison post hoc test was employed to determine the significance of changes between control adhesion and adhesion produced by trypsin in the ex vivo study. In all cases a P-value less than 0.05 was taken to be indicative of statistical significance.
Drugs and reagents
All chemicals were purchased from Sigma-Aldrich, Dorset, UK unless otherwise stated. 51Cr was from Amersham Bioscience International Plc., Buckinghamshire, UK; Australia foetal bovine serum and RPMI-1640 were from Invitrogen Ltd, Paisley, UK; normal rabbit serum and streptavidin-horse radish peroxide were from Vector Lab., Peterborough, UK. Anti-P-selectin goat polyclonal antibody and anti-ICAM-1 goat polyclonal antibody were from Santa Cruz Biotech., CA, USA and R & D Systems, Oxford, UK, respectively. Biotinylated rabbit anti-goat secondary antibody was from DAKO Ltd, Cambridgeshire, UK.
Catalase and sodium azide were dissolved in saline on a daily basis. Anti-ICAM-1 goat polyclonal antibody, anti-P-selectin goat polyclonal antibody and biotinylated rabbit anti-goat secondary antibody were diluted with antibody diluent (DAKO Ltd, Cambridgeshire, UK) on a daily basis. Normal rabbit serum was diluted daily in PBS. PAF, SOD and trypsin were dissolved in saline and frozen at −20°C as aliquots. Aliquots were thawed and freshly diluted daily with saline. TNFα and PMA were dissolved in PBS and absolute ethanol, respectively, frozen aliquots were thawed and fresh dilutions were prepared daily with saline. At the highest concentration of PMA, the final ethanol concentration was 1%. Trypan blue was dissolved in saline and Toluidine blue was dissolved in distilled water and stored at room temperature. Luminol solution was prepared daily in 2 M NaOH (2.5%) and PBS.
Results
Flow cytometric analysis of isolated spleen cells
Isolated cells from three separate mice contained a similar proportion of CD4+ T lymphocytes (24.2±4.4%), B-220+B lymphocytes (24.2±7.7%) and CD11b+ myeloid cells (21.6±3.1%). The remaining 20% of unlabelled cells is likely to be composed of dendritic cells and some debris. Analysis of the cell types adhering after 30 min revealed that all three cell types were present and at approximately the same proportions as was found in the isolated spleen cells (31.2±5.6% for B lymphocytes, 25.5±6.9% for T lymphocytes and 19±0.9% for myeloid cell; n=3; P>0.05 compared to isolated spleen cells).
Effect of drug treatments on lymphocyte adhesion
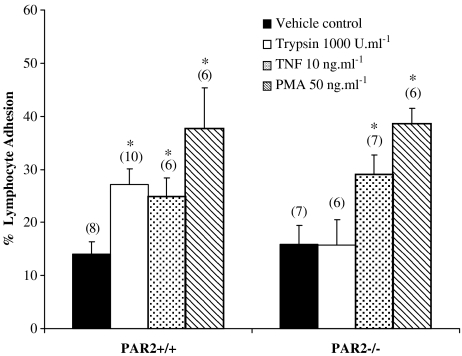
Basal lymphocyte adhesion in arteries from PAR-2+/+ mice was similar to that observed in arteries from PAR-2−/− mice. Pretreating thoracic aorta with trypsin significantly increased lymphocyte adhesion in PAR-2+/+ but not the PAR-2−/− mice. PMA and TNFα, however, significantly enhanced lymphocyte adhesion in both PAR-2+/+ and PAR-2−/− mice (Figure 1). Incubation of aortic segments with trypsin (1000 U ml−1) for 30 min did not cause any discernable damage to the endothelial layer as there was no increase in Toluidine Blue staining compared to saline-treated control arteries (approximately 70% endothelium was present in all six aortic segments studied).
Figure 1.
Effect of drug treatments on lymphocyte adhesion in vitro. Effect of trypsin (1000 U ml−1, 30 min), TNFα (10 ng ml−1, 60 min) and PMA (50 ng ml−1, 30 min) on adhesion of lymphocytes to thoracic aorta isolated from PAR-2+/+ and PAR-2−/− mice. Numbers in parentheses indicate n numbers. *Indicates P<0.05 vs vehicle control.
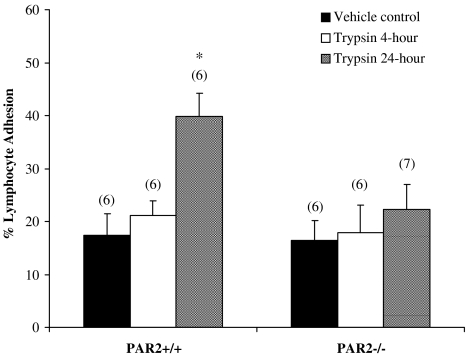
In PAR-2+/+ mice, adhesion of lymphocytes to thoracic aorta was significantly elevated 24-h after an intravenous injection of trypsin (bolus dose of 1 U g−1) (Figure 2). In contrast, PAR-2−/− mice receiving a bolus dose of trypsin intravenously did not show any significant change in lymphocyte adhesion at either 4 or 24 h after the injection. The basal lymphocyte adhesion was not significantly different between PAR-2+/+ and PAR-2−/− mice.
Figure 2.
Effect of drug treatments on lymphocyte adhesion ex vivo. Effect of intravenous trypsin injection (1 U g−1, 0.05 ml per 25 g) on adhesion of autologous lymphocytes to PAR-2+/+ and PAR-2−/− mice thoracic aorta. Adhesion was studied 4 or 24 h post-trypsin injection. Numbers in parentheses indicate n numbers. *Indicates P<0.01 vs vehicle control.
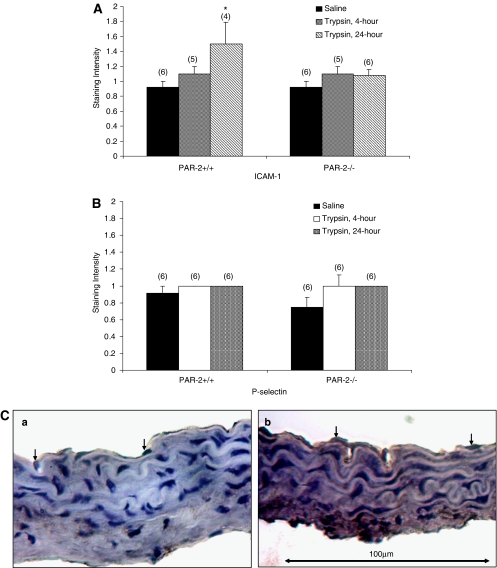
Effect of trypsin on P-selectin and ICAM-1 expression
Following the adhesion assay, arteries were fixed in formal saline and subsequently used for immunocytochemical examination of P-selectin and ICAM-1 expression. All segments showed staining for P-selectin and ICAM-1 on the endothelium compared to negative controls (without primary antibody). A significantly higher intensity of staining for ICAM-1 was observed in thoracic aorta isolated from PAR-2+/+ mice compared to PAR-2−/− mice 24 h after the injection of trypsin (Figure 3A). No difference was found in P-selectin staining on the thoracic aortae of PAR-2+/+ or PAR-2−/− mice following trypsin injection either 4 or 24 h earlier (Figure 3B). In arteries from in vitro studies, no differences were detected in the degree of P-selectin or ICAM-1 staining in any segments with the exception of TNFα in the PAR-2+/+ thoracic aorta, which showed a significant increase in P-selectin staining (data not shown).
Figure 3.
Adhesion molecule expression on endothelium. Quantification of ICAM-1 (A) or P-selectin (B) staining on thoracic aorta isolated from PAR-2+/+ and PAR-2−/− mice subjected to lymphocyte adhesion assay (ex vivo study). Arteries were isolated from mice injected with either saline (vehicle control) or trypsin (4 or 24 h). (C) photomicrographs showing the up-regulation of ICAM-1 staining in the thoracic aorta isolated from the PAR-2+/+ mice 24 h after trypsin injection compared with the vehicle control segments. Arrows indicate the endothelial nucleus. Numbers in parentheses indicate n numbers. *Indicates P<0.05 vs vehicle control.
Lymphocyte ROS generation
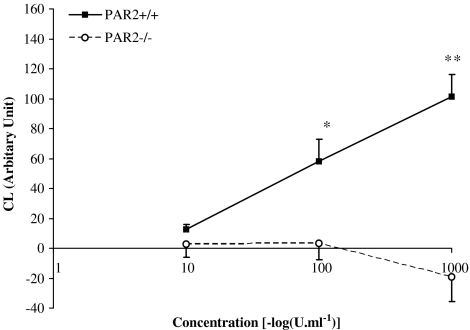
The chemiluminescence response from lymphocytes treated with saline was the same in PAR-2+/+ and PAR-2−/− mice (arbitrary unit; 672±60 vs 794±113, respectively, P>0.05). PMA and PAF produced a similar concentration-dependent chemiluminescence response in both PAR-2+/+ and PAR-2−/− mouse lymphocytes (maximum response above basal was 1155±223 in PAR 2+/+ vs 1142±136 in PAR 2−/− for PMA and 622±194 vs 1124±403 for PAF; P>0.05). Trypsin induced a concentration-dependent chemiluminescence response in lymphocytes isolated from PAR-2+/+, but not PAR-2−/− mice (Figure 4). The magnitude of the chemiluminescence signal induced by trypsin was much smaller than those induced by PMA and PAF.
Figure 4.
Effect of PAR-2 receptor knockout on the chemiluminescent response to trypsin. The Figure shows the total chemiluminescence (CL) signal generated by PAR-2+/+ (n=6) and PAR-2−/− (n=6) mouse lymphocytes stimulated with varying concentrations of trypsin. The values shown are corrected for CL signals generated by saline alone. * and **Indicate P<0.05 and <0.001 vs PAR-2−/−, respectively.
Effect of scavengers on PMA-induced ROS generation
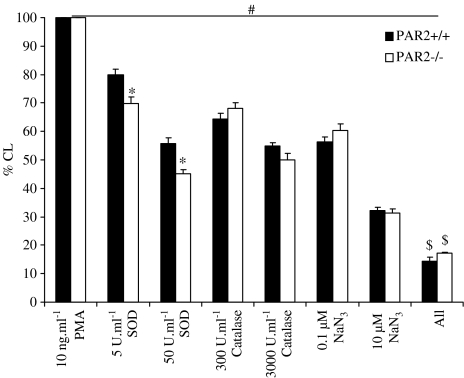
PMA (10 ng ml−1) generated a similar degree of chemiluminescence in PAR-2+/+ and PAR-2−/− mouse lymphocytes (arbitrary units after correction with saline; 784±75 vs 791±264 in PAR-2+/+ and PAR-2−/−, respectively, n=6; P>0.05). SOD, catalase and sodium azide all produced a concentration-dependent inhibition of the PMA-induced chemiluminescence signal. However, SOD produced a significantly greater inhibition in the PAR-2−/− mouse lymphocytes compared to the PAR-2+/+. Co-administration of all three scavengers (at the highest concentrations used in this study; 50 U ml−1 SOD+3000 U ml−1 catalase+10−5 M sodium azide) produced a significantly greater, but not complete, inhibition of the chemiluminescence signal generated by 10 ng ml−1 of PMA compared to the individual scavengers alone (arbitrary units; 121±8 and 168±15 in PAR-2+/+ and PAR-2−/−, respectively) (Figure 5).
Figure 5.
Effect of ROS scavengers on PMA-induced chemiluminescence (CL). The effects of SOD, catalase and/or sodium azide on CL signal generated by lymphocytes from PAR-2+/+ (n=6) and PAR-2−/− (n=6) mice, stimulated with 10 ng ml−1 PMA . ‘All' indicates 50 U ml−1 of SOD+3000 U ml−1 catalase+10−5 M sodium azide. #indicates P<0.05 compared to 10 ng ml−1 PMA, * indicates P<0.01 compared to PAR-2+/+, and $ indicates P<0.05 compared to 50 U ml−1 of SOD, to 3000 U ml−1 catalase and to 10−5 M sodium azide.
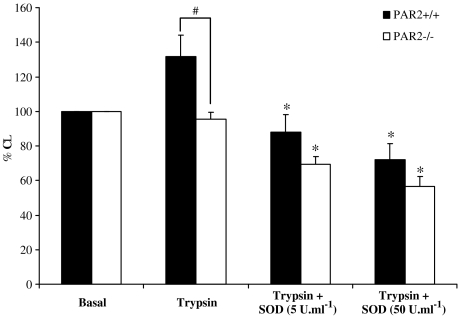
Effect of SOD on trypsin-induced ROS generation
Basal chemiluminescence in the absence of trypsin stimulation was not significantly different between PAR-2+/+ and PAR-2−/− mouse lymphocytes (arbitrary unit was 736±149 and 1133±332 for PAR-2+/+ and PAR-2−/−, respectively, P>0.05). Trypsin (1000 U ml−1) caused an increase in chemiluminescence response in PAR-2+/+, but not PAR-2−/− mouse lymphocytes (Figure 6). SOD dose dependently inhibited the chemiluminescence signal generated by trypsin in the PAR-2+/+ mouse lymphocytes. SOD also dose dependently inhibited the chemiluminescence signal generated from PAR-2−/− mouse lymphocytes in the presence of trypsin to a level below basal and the inhibitory profile of SOD on trypsin-induced chemiluminescence signal was similar in both PAR-2+/+ and PAR-2−/− mouse lymphocytes.
Figure 6.
Effect of SOD on trypsin-induced chemiluminescence. The effect of SOD on the chemiluminescence signal generated by lymphocytes from PAR2+/+ (n=8–14) and PAR2−/− (n=8–14) mice, stimulated with 1000 U ml−1 trypsin. Basal is the chemiluminescence response to saline (unstimulated) expressed as 100%. *Indicates P<0.05 compared to trypsin 1000 U ml−1 of respective PAR-2 mice and #indicates P<0.05.
Discussion
The present study has shown that trypsin increases the adhesion of lymphocytes to the luminal surface of mouse thoracic aorta when applied exogenously or administered intravenously 24 h beforehand and that this effect is not owing to damage to the integrity of the endothelium. Trypsin also induced ROS generation from isolated mouse lymphocytes. The fact that these effects of trypsin were absent in the PAR-2−/− mice has provided evidence for PAR-2-dependent pro-inflammatory effects. This corroborates other in vivo studies where intraplantar administration of trypsin or PAR-2 activating peptides into rat and mouse hind paw induced a profound acute inflammatory response characterized by granulocyte infiltration (Vergnolle, 1999; Tae et al., 2003).
PAR-2 deletion itself did not significantly modify the basal lymphocyte adhesion or ROS generation. This suggests that PAR-2 does not have an important role in regulating lymphocyte adhesion and ROS generation in the mouse under physiological conditions. PAR-2 may, however, play a role under pathophysiological conditions, as in a previous study, PAR-2−/− mice subjected to inflammation by mild tissue trauma exhibited an acute reduction in P-selectin-mediated leucocyte rolling, which was not sustained beyond 30 min after injury (Lindner et al., 2000).
The ability of trypsin to enhance lymphocyte adhesion was observed in PAR-2+/+ mice in both the in vitro and the ex vivo studies. This is in contrast with a previous study, which suggested that trypsin pretreatment may have an anti-adhesive effect by inhibiting soluble L-selectin binding to bovine aortic endothelial cells (Giuffre et al., 1997). In the present study, the failure of trypsin to induce lymphocyte adhesion in PAR-2−/− mice suggests that the effect of trypsin on lymphocyte adhesion is PAR-2 mediated. This has also eliminated the possible involvement of PAR-1 and PAR-4 where high concentrations of trypsin have been shown to be non-selective for both PAR-1 and PAR-2 (McLean et al., 2002), and trypsin is also an endogenous agonist for PAR-4 (Xu et al., 1998). PAR-2-mediated leucocyte rolling has previously been suggested to be P-selectin dependent at the initial stage (Lindner et al., 2000), although P-selectin expression is not a prerequisite for firm adhesion. In the present study, there was no change in P-selectin expression. This is perhaps not surprising as the adhesion assay was measuring firm adhesion at a 30-min-time point, a time at which P-selectin expression in stimulated endothelium is thought to have waned following early upregulation (Hattori et al., 1989; Collins et al., 1993). The lack of change in ICAM-1 expression in trypsin-treated arteries in the in vitro experiment has also ruled out the possibility of ICAM-1 translocation from an intracellular store to the cell surface. However, the delayed effect of trypsin (24 h after injection) on lymphocyte adhesion could be due to upregulation of ICAM-1, but not P-selectin expression on the endothelium. The increased expression of ICAM-1 also correlated with the time course required for ICAM-1 de novo protein synthesis. An alternative explanation for enhanced adhesion following artery stimulation with trypsin is via PAR-2-mediated expression of other adhesion molecules, such as E-selectin (Ishikawa et al., 1993; Seeliger et al., 2003), or release of mediators relevant to lymphocyte adhesion such as PAF, which activates β2 integrins to promote firm adhesion (Vergnolle et al., 1999).
TNFα and PMA were employed in the present study as a positive control acting through a PAR-2-independent mechanism. In contrast to trypsin, both stimulants significantly enhanced lymphocyte adhesion in both PAR-2+/+ and PAR-2−/− mouse thoracic aorta. The ability of TNFα to increase leucocyte-endothelium interactions has been well established in vitro (Pichyangkul et al., 1988; Aparicio et al., 1996) and in vivo (Eriksson et al., 2000; Thorlacius et al., 2000; Miller et al., 2005). The pro-adhesive effect of PMA has also been demonstrated in various studies, albeit using different types of inflammatory cells and species (Gudewicz et al., 1989; Lane et al., 1989; Itoh et al., 2003).
The luminol molecule reacts with ROS to form excited aminophthalate anions that decay to the ground state with the release of energy in the form of light, which is measured as chemiluminescence (Briheim et al., 1984; Lundqvist and Dahlgren, 1996). The current study has shown that mouse lymphocytes are capable of generating a reproducible increase in the chemiluminescence signal when stimulated with PMA, PAF or trypsin. Trypsin induced a concentration-dependent increase in the chemiluminescence signal in PAR-2+/+ lymphocytes and this ROS-generating effect of trypsin was abolished in the lymphocytes isolated from the PAR-2-deficient mice. This supports the view that activation of PAR-2 is responsible for the ROS generation in response to trypsin seen in the wild-type mice. The present study has further demonstrated that trypsin is capable of inducing ROS release from another leucocyte subtype, the lymphocyte, in addition to neutrophils (Williams et al., 1986), eosinophils (Miike et al., 2001; Bolton et al., 2003) and lung fibroblasts (Aoshiba et al., 2001). However, it should be noted that our spleen preparations contain some 15–20% of myeloid cells and the contribution of these cells to the total chemiluminescence signal is unknown. Other studies have also provided indirect evidence for a stimulatory role for trypsin by demonstrating an inhibitory effect of trypsin inhibitors (such as α1-antitrypsin and soybean trypsin inhibitor) on human neutrophil ROS production (Bucurenci et al., 1992; Nishijima et al., 1992). In contrast, a recent study in peritoneal macrophages has reported an inhibitory effect of trypsin on the release of ROS (Bryniarski et al., 2003). Moreover, previous studies in human neutrophils have shown that the urinary trypsin inhibitors, gabexate mesylate and ulinastatin, inhibit extracellular release of ROS but enhance the generation of intracellular ROS (Nishijima et al., 1992). This may suggest that the source of ROS in trypsin-treated lymphocytes in the present study is more likely to be through extracellular release rather than of intracellular origin. Furthermore, extracellular ROS are the primary bystander substrates for luminol and predominate in the early chemiluminescence response whereas detection of ROS of intracellular origin has been suggested to be limited by the diffusion of luminol molecules into the cells (Stevens et al., 1978; Briheim et al., 1984).
PMA (Rabesandratana et al., 1992; Demiryurek et al., 1994; Lundqvist and Dahlgren, 1996) and PAF (Takahashi et al., 1991; Kato et al., 2002) have been well documented to stimulate the production of ROS from leucocytes. In the present study, both PMA and PAF produced concentration-dependent increases in ROS generation in PAR-2+/+ mice lymphocytes, which were not modified by PAR-2 deficiency as shown by their responses in lymphocytes from PAR-2−/− mice. These important results demonstrate that the isolated cells from PAR-2−/− are capable of stimulation and chemiluminescence generation and strengthens the conclusion that it is activation of the PAR-2 receptor by trypsin which leads to generation of chemiluminescence in the PAR-2-+/+ mouse.
The magnitude of the chemiluminescence response to trypsin was much smaller than those induced by PMA and PAF. This may be related to PAR-2 only being expressed on T lymphocytes and not B lymphocytes, which made up 25% of the spleen cell population (Mari et al., 1996; Hou et al., 1998). However, anti-trypsin present in the serum-containing lymphocyte suspension may also have inhibited the action of trypsin.
In the present study, SOD (a superoxide radical scavenger), catalase (a hydrogen peroxide scavenger) and sodium azide (a myeloperoxidase inhibitor) all had a dose-dependent inhibitory effect on chemiluminescence generation as has been observed in porcine leucocytes (Demiryurek et al., 1994). However, SOD produced a significantly greater inhibition of the chemiluminescence signal generated from the PAR-2−/− lymphocytes compared to the PAR-2+/+ mouse lymphocytes, suggesting a greater proportion of superoxide formation in PAR-2−/− mice although this requires further investigation. Complete inhibition of the chemiluminescence signal was not achieved using a combination of SOD, catalase and sodium azide, and so other radicals may contribute to the chemiluminescence signal or part of the signal may be intracellular where scavenger molecules may have trouble gaining access (Stevens et al., 1978). The ability of cell-free medium to produce a chemiluminescence response could be explained by the presence of glucose, phenol red, divalent cations (Mg2+ and Ca2+), protein (bovine serum in medium), vitamins and amino acids in the medium (Glette et al., 1982; Hastings et al., 1982). This may also explain the inhibitory effect of SOD in trypsin-treated PAR-2−/− mouse lymphocytes, which may be due to an action on the ROS produced from the medium and unstimulated lymphocytes (a chemiluminescence signal was observed in saline-treated lymphocytes).
To further verify the involvement of PAR-2 in superoxide production, we examined the effect of SOD on trypsin-induced ROS generation from PAR-2 mouse lymphocytes. Although SOD dose dependently inhibited trypsin-stimulated ROS generation from the PAR-2+/+ mouse lymphocytes, in agreement with a previous study in human eosinophils (Miike et al., 2001), it also exerted an inhibitory effect in PAR-2−/− mice, despite a lack of trypsin stimulation. This implies that a basal release of superoxide from the PAR-2−/− lymphocytes contributes to the chemiluminescence detected.
To our knowledge, these data represent novel findings. Using both in vitro and ex vivo assays, we have demonstrated that trypsin, employed as a PAR-2 agonist, is capable of increasing lymphocyte adhesion in intact mouse arteries through a PAR-2-dependent pathway. The mechanism underlying the delayed effect of trypsin in lymphocyte adhesion observed in the ex vivo study is suggested to involve an increased expression of ICAM-1 on the endothelium whereas the mechanism underlying the acute effect of trypsin illustrated in the in vitro experiments is yet to be determined. In addition, the present study has also provided evidence that activation of murine lymphocytes by activation of PAR-2 with trypsin induces ROS generation. In conclusion, PAR-2 activation by trypsin appears to mediate the pro-inflammatory response in the mouse model by enhancing the adhesion of lymphocytes to arteries and ROS generation from isolated lymphocytes.
Acknowledgments
We thank Professor R Plevin for the provision of PAR-2 genetic-modified mice. We are also grateful to Dr AR McPhaden for his assistance in immunocytochemistry and Dr C Rush for assistance with flow cytometry experiments.
Abbreviations
- PAF
platelet-activating factor
- PAR
protease-activated receptor
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Conflict of interest
The authors state no conflict of interest.
References
- Aoshiba K, Yasuda K, Yasui S, Tamaoki J, Nagai A. Serine proteases increase oxidative stress in lung cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L556–L564. doi: 10.1152/ajplung.2001.281.3.L556. [DOI] [PubMed] [Google Scholar]
- Aparicio CL, Berthiaume F, Chang CC, Yarmush ML. Tumor necrosis factor-alpha (TNF-alpha) induces a reversible, time- and dose-dependent adhesion of progenitor T cells to endothelial cells. Mol Immunol. 1996;33:671–680. doi: 10.1016/0161-5890(96)00013-2. [DOI] [PubMed] [Google Scholar]
- Bolton SJ, Mcnulty CA, Thomas RJ, Hewitt CRA, Wardlaw AJ. Expression of and functional responses to protease-activated receptors on human eosinophils. J Leukoc Biol. 2003;74:60–68. doi: 10.1189/jlb.0702351. [DOI] [PubMed] [Google Scholar]
- Briheim G, Stendahl O, Dahlgren C. Intracellular and extracellular events in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1984;45:1–5. doi: 10.1128/iai.45.1.1-5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryniarski K, Maresz K, Szczepanik M, Ptak M, Ptak W. Modulation of macrophage activity by proteolytic enzymes. Differential regulation of IL-6 and reactive oxygen intermediates (ROIs) synthesis as a possible homeostatic mechanism in the control of inflammation. Inflammation. 2003;27:333–340. doi: 10.1023/b:ifla.0000006701.52150.43. [DOI] [PubMed] [Google Scholar]
- Bucurenci N, Blake DR, Chidwick K, Winyard PG. Inhibition of neutrophil superoxide production by human plasma alpha-1-antitrypsin. FEBS Lett. 1992;300:21–24. doi: 10.1016/0014-5793(92)80156-b. [DOI] [PubMed] [Google Scholar]
- Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- Collins PW, Macey MG, Cahill MR, Newland AC. Von-Willebrand factor release and P-selectin expression is stimulated by thrombin and trypsin but not IL-1 in cultured human endothelial cells. Thromb Haemost. 1993;70:346–350. [PubMed] [Google Scholar]
- Colognato R, Slupsky JR, Jendrach M, Burysek L, Syrovets T, Simmet T. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood. 2003;102:2645–2652. doi: 10.1182/blood-2002-08-2497. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, et al. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24:4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano BP, Cheung MW, Santulli RJ, Fung-Leung WP, Ngo K, Ye RD, et al. Cardiovascular responses mediated by protease-activated receptor-2 (PAR-2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR-2 or PAR-1. J Pharmacol Exp Ther. 1999;288:671–678. [PubMed] [Google Scholar]
- D'Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, et al. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J Histochem Cytochem. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- Demiryurek AT, Wainwright CL, Wadsworth RM, Kane KA. Characterization of a method for the detection of drugs with free radical scavenging activity using porcine leukocytes. J Pharmacol Toxicol Methods. 1994;32:35–40. doi: 10.1016/1056-8719(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Eriksson EE, Werr J, Guo YC, Thoren P, Lindbom L. Direct observations in vivo on the role of endothelial selectins and alpha(4) integrin in cytokine-induced leukocyte-endothelium interactions in the mouse aorta. Circ Res. 2000;86:526–533. doi: 10.1161/01.res.86.5.526. [DOI] [PubMed] [Google Scholar]
- Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, et al. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffre L, Cordey AS, Monai N, Tardy Y, Schapira M, Spertini O. Monocyte adhesion to activated aortic endothelium: role of L-selectin and heparan sulfate proteoglycans. J Cell Biol. 1997;136:945–956. doi: 10.1083/jcb.136.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glette J, Solberg CO, Lehmann V. Factors influencing human polymorphonuclear leukocyte chemiluminescence. Acta Pathol Microbiol Immunol Scand. 1982;90:91–95. doi: 10.1111/j.1699-0463.1982.tb01423.x. [DOI] [PubMed] [Google Scholar]
- Gudewicz PW, Weaver MB, Delvecchio PJ, Saba TM. Phorbol-myristate acetate treated endothelium stimulates polymorphonuclear leukocyte adhesion and superoxide secretion. J Lab Clin Med. 1989;113:708–716. [PubMed] [Google Scholar]
- Hallenbeck JM, Hansson GK, Becker KJ. Immunology of ischemic vascular disease: plaque to attack. Trends Immunol. 2005;26:550–556. doi: 10.1016/j.it.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hastings MJG, Petricevic I, Williams AJ, Cole PJ, Easmon CSF. The effect of culture media on the production and measurement of luminol-dependent chemiluminescence. Br J Exp Pathol. 1982;63:147–153. [PMC free article] [PubMed] [Google Scholar]
- Hattori R, Hamilton KK, Fugate RD, Mcever RP, Sims PJ. Stimulated secretion of endothelial Von Willebrand factor is accompanied by rapid redistribution to the cell-surface of the intracellular granule membrane-protein GMP-140. J Biol Chem. 1989;264:7768–7771. [PubMed] [Google Scholar]
- Hou L, Howells GL, Kapas S, Macey MG. The protease-activated receptors and their cellular expression and function in blood-related cells. Br J Haematol. 1998;101:1–9. doi: 10.1046/j.1365-2141.1998.00696.x. [DOI] [PubMed] [Google Scholar]
- Howells GL, Macey MG, Chinni C, Hou L, Fox MT, Harriott P, et al. Proteinase-activated receptor-2: Expression by human neutrophils. J Cell Sci. 1997;110:881–887. doi: 10.1242/jcs.110.7.881. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Imura A, Tanaka K, Shirane H, Okuma M, Uchiyama T. E-selectin and vascular cell adhesion molecule-1 mediate adult T-cell leukemia-cell adhesion to endothelial cells. Blood. 1993;82:1590–1598. [PubMed] [Google Scholar]
- Itoh M, Omi H, Okouchi M, Imaeda K, Shimizu M, Fukutomi T, et al. The mechanisms of inhibitory actions of gliclazide on neutrophils-endothelial cells adhesion and surface expression of endothelial adhesion molecules mediated by a high glucose concentration. J Diabetes Complicat. 2003;17:22–26. doi: 10.1016/s1056-8727(01)00219-7. [DOI] [PubMed] [Google Scholar]
- Kato M, Kimura H, Motegi Y, Tachibana A, Minakami H, Morikawa A, et al. Platelet-activating factor activates two distinct effector pathways in human eosinophils. J Immunol. 2002;169:5252–5259. doi: 10.4049/jimmunol.169.9.5252. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kuroda R, Minami T, Kataoka K, Taneda M. Increased vascular permeability by a specific agonist of protease-activated receptor-2 in rat hindpaw. Br J Pharmacol. 1998;125:419–422. doi: 10.1038/sj.bjp.0702063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S, Wadsworth RM, McPhaden AR, Wainwright CL. A rapid, quantitative method for measuring leukocyte adhesion to normal and balloon-injured arteries in vitro. J Immunol Methods. 2000;244:153–162. doi: 10.1016/s0022-1759(00)00266-0. [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI, Brauner JA. Flow cytometric analysis of lymphocyte subpopulations in the spleen and thymus of mice exposed to an acute immunosuppressive dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Environ Res. 1990;52:146–154. doi: 10.1016/s0013-9351(05)80249-x. [DOI] [PubMed] [Google Scholar]
- Klein JB, Witonsky SG, Ahmed SA, Holladay SD, Gogal RM, Jr, Link L, et al. Impact of different cell isolation techniques on lymphocyte viability and function. J Immunoassay Immunochem. 2006;27:61–76. doi: 10.1080/15321810500403755. [DOI] [PubMed] [Google Scholar]
- Lane TA, Lamkin GE, Wancewicz E. Modulation of endothelial cell expression of intercellular adhesion molecule-1 by protein kinase-C activation. Biochem Biophys Res Commun. 1989;161:945–952. doi: 10.1016/0006-291x(89)91334-x. [DOI] [PubMed] [Google Scholar]
- Lindner JR, Kahn ML, Coughlin SR, Sambrano GR, Schauble E, Bernstein D, et al. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol. 2000;165:6504–6510. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- Lundqvist H, Dahlgren C. Isoluminol-enhanced chemiluminescence: a sensitive method to study the release of superoxide anion from human neutrophils. Free Radical Biol Med. 1996;20:785–792. doi: 10.1016/0891-5849(95)02189-2. [DOI] [PubMed] [Google Scholar]
- Mari B, Guerin S, Far DF, Breitmayer JP, Belhacene N, Peyron JF, et al. Thrombin and trypsin-induced Ca2+ mobilization in human T cell lines through interaction with different protease-activated receptors. FASEB J. 1996;10:309–316. doi: 10.1096/fasebj.10.2.8641564. [DOI] [PubMed] [Google Scholar]
- Mclean PG, Aston D, Sarkar D, Ahluwalia A. Protease-activated receptor-2 activation causes EDHF-like coronary vasodilation – selective preservation in ischemia/reperfusion injury: Involvement of lipoxygenase products, VR1 receptors, and C-fibers. Circ Res. 2002;90:465–472. doi: 10.1161/hh0402.105372. [DOI] [PubMed] [Google Scholar]
- Miike S, McWilliam AS, Kita H. Trypsin induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. J Immunol. 2001;167:6615–6622. doi: 10.4049/jimmunol.167.11.6615. [DOI] [PubMed] [Google Scholar]
- Miller AM, McPhaden AR, Preston A, Wadsworth RM, Wainwright CL. TNF alpha increases the inflammatory response to vascular balloon injury without accelerating neointimal formation. Atherosclerosis. 2005;179:51–59. doi: 10.1016/j.atherosclerosis.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Nishijima J, Hiraoka N, Murata A, Oka Y, Kitagawa K, Tanaka N, et al. Protease inhibitors (gabexate mesylate and ulinastatin) stimulate intracellular chemiluminescence in human neutrophils. J Leukoc Biol. 1992;52:262–268. doi: 10.1002/jlb.52.3.262. [DOI] [PubMed] [Google Scholar]
- Nystedt S, Emilsson IE, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystedt S, Ramakrishnan V, Sundelin J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells – comparison with the thrombin receptor. J Biol Chem. 1996;271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- Pichyangkul S, Schick D, Schober W, Dixon G, Khan A. Increased expression of adhesive proteins on leukocytes by TNF-alpha. Exp Hematol. 1988;16:588–593. [PubMed] [Google Scholar]
- Rabesandratana H, Fournier AM, Chateau MT, Serre A, Dornand J. Increased oxidative metabolism in PMA-activated lymphocytes – A flow cytometric study. Int J Immunopharmacol. 1992;14:895–902. doi: 10.1016/0192-0561(92)90089-4. [DOI] [PubMed] [Google Scholar]
- Seeliger S, Derian CK, Vergnolle N, Bunnett NW, Nawroth R, Schmelz M, et al. Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammation in vivo. FASEB J. 2003;17:1871–1885. doi: 10.1096/fj.02-1112com. [DOI] [PubMed] [Google Scholar]
- Shresta S, Pham CTN, Thomas DA, Graubert TA, Ley TJ. How do cytotoxic lymphocytes kill their targets? Curr Opin Immunol. 1998;10:581–587. doi: 10.1016/s0952-7915(98)80227-6. [DOI] [PubMed] [Google Scholar]
- Stevens P, Winston DJ, Vandyke K. In vitro evaluation of opsonic and cellular granulocyte function by luminol-dependent chemiluminescence – utility in patients with severe neutropenia and cellular deficiency states. Infect Immun. 1978;22:41–51. doi: 10.1128/iai.22.1.41-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tae J, Han SW, Yoo JY, Kim JA, Kang OH, Baek OS, et al. Anti-inflammatory effect of Lonicera japonica in proteinase-activated receptor 2-mediated paw edema. Clin Chim Acta. 2003;330:165–171. doi: 10.1016/s0009-8981(03)00017-2. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yoshikawa T, Naito Y, Tanigawa T, Yoshida N, Kondo M. Role of platelet-activating-factor (PAF) in superoxide production by human polymorphonuclear leukocytes. Lipids. 1991;26:1227–1230. doi: 10.1007/BF02536537. [DOI] [PubMed] [Google Scholar]
- Thorlacius H, Vollmar B, Guo Y, Mak TW, Pfreundschuh MM, Menger MD, et al. Lymphocyte function antigen 1 (LFA-1) mediates early tumour necrosis factor alpha-induced leucocyte adhesion in venules. Br J Haematol. 2000;110:424–429. doi: 10.1046/j.1365-2141.2000.02162.x. [DOI] [PubMed] [Google Scholar]
- Varda-Bloom N, Leor J, Ohad DG, Hasin Y, Amar M, Fixler R, et al. Cytotoxic T lymphocytes are activated following myocardial infarction and can recognize and kill healthy myocytes in vitro. J Mol Cell Cardiol. 2000;32:2141–2149. doi: 10.1006/jmcc.2000.1261. [DOI] [PubMed] [Google Scholar]
- Vergnolle N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol. 1999;163:5064–5069. [PubMed] [Google Scholar]
- Vergnolle N, Hollenberg MD, Sharkey KA, Wallace JL. Characterization of the inflammatory response to proteinase-activated receptor-2 (PAR(2))-activating peptides in the rat paw. Br J Pharmacol. 1999;127:1083–1090. doi: 10.1038/sj.bjp.0702634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TK, Hung HDT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Williams JD, Topley N, Alobaidi HM, Harber MJ. Activation of human polymorphonuclear leukocytes by particulate zymosan is related to both its major carbohydrate components – glucan and mannan. Immunology. 1986;58:117–124. [PMC free article] [PubMed] [Google Scholar]
- Xu WF, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, et al. Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci USA. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ysebaert DK, De Greef KE, De Beuf A, Van Rompay AR, Vercauteren S, Persy VP, et al. T cells as mediators in renal ischemia/reperfusion injury. Kidney Int. 2004;66:491–496. doi: 10.1111/j.1523-1755.2004.761_4.x. [DOI] [PubMed] [Google Scholar]