Abstract
Downregulation of vascular endothelial constitutive nitric oxide synthase (ecNOS) contributes to the vascular hyporesponsiveness in sepsis. Although coadministration of the potent vasodilatory peptide adrenomedulin (AM) and the newly discovered AM binding protein (AMBP-1) maintains cardiovascular stability and reduces mortality in sepsis, it remains unknown whether AM/AMBP-1 prevents endothelial cell dysfunction. To investigate this possibility, we subjected adult male rats to sepsis by cecal ligation and puncture (CLP), with or without subsequent intravenous administration of the combination of AM (12 μg/kg) and AMBP-1 (40 μg/kg). Thoracic aortae were harvested 20 h after CLP (i.e., the late stage of sepsis) and endothelium-dependent vascular relaxation was determined by the addition of acetylcholine (ACh) in an organ bath system. In addition, ecNOS gene and protein expression was assessed by RT-PCR and immunohistochemistry, respectively. The results indicate that ACh-induced (i.e., endothelium-dependent) vascular relaxation was significantly reduced 20 h after CLP. Administration of AM/AMBP-1 prevented the reduction of vascular relaxation. In addition, ecNOS gene expression in aortic and pulmonary tissues was downregulated 20 h after CLP and AM/AMBP-1 attenuated such a reduction. Moreover, the decreased ecNOS staining in thoracic aortae of septic animals was prevented by the treatment with AM/AMBP-1. These results, taken together, indicate that AM/AMBP-1 preserves ecNOS and prevents reduced endothelium-dependent vascular relaxation (i.e., endothelial cell dysfunction) in sepsis. In light of our recent finding that AM/AMBP-1 improves organ function and reduces mortality in sepsis, it is most likely that the protective effect of these compounds on ecNOS is a mechanism responsible for the salutary effect of AM/AMBP-1 in sepsis.
INTRODUCTION
Sepsis, septic shock, and multiple organ failure continue to be the most common causes of death in noncardiac intensive care units (1–3). Despite advances in the management of trauma victims, the incidence of sepsis and septic shock has increased significantly over the past two decades (3–6). Human adrenomedullin (AM) is a 52–amino acid peptide which was first isolated from pheochromocytomas by Kitamura et al. and reported in 1993 (7). Rat AM has 50 amino acid residues, with two amino acid deletions and six substitutions compared with human AM (8). Circulating levels of AM increase significantly in patients with septic shock (9–11) and systemic inflammatory response syndrome (12), and after major surgery (13). Upregulation of AM can also be observed after administration of endotoxin in vivo as well as in vitro in vascular smooth muscle cells and macrophages (14–20). Our recent studies have demonstrated that the small intestine is a major source of AM production and release during sepsis (21). Studies by Elsasser et al. (22) have demonstrated the presence of a specific AM binding protein (120 and/or 140 kDa) in mammalian blood. Pio et al. purified the binding protein, which they named AMBP-1, and discovered that AMBP-1 is identical to human complement factor H (23). The primary site of AMBP-1 (factor H) biosynthesis is the liver (24–26), and the only extrahepatic source of AMBP-1 in humans under in vivo conditions is the small amount produced in the lungs (27). The finding that AMBP-1 potentiates AM-induced cAMP accumulation in cultured Rat-2 fibroblast cells (23) suggests that AMBP-1 plays an important role in AM-induced vascular relaxation. Thus, circulating AMBP-1 can positively affect the bioactivity of AM under normal as well as disease conditions (23). To this end, our results have indicated that AMBP-1 improves vascular responsiveness to AM stimulation (28). Moreover, we have shown that AMBP-1 production is decreased in sepsis, and this reduction in AMBP-1 appears to be responsible for the vascular AM hyporesponsiveness observed in the hypodynamic phase (28). Moreover, coadministration of AM and AMBP-1 downregulates proinflammatory cytokines (29), maintains cardiovascular stability (30), prevents endothelial cell apoptosis (31) in sepsis and reduces sepsis-induced mortality (30).
Studies have indicated that downregulation of vascular endothelial constitutive nitric oxide synthase (ecNOS) contributes to vascular hyporesponsiveness in sepsis (32–34). Although administration of AM/AMBP-1 is beneficial in sepsis, whether AM/AMBP-1 prevents endothelial cell dysfunction under such conditions is not known. We therefore hypothesized that AM/AMBP-1 preserves endothelium-dependent vascular relaxation by preventing ecNOS downregulation in sepsis. Endothelium-dependent vascular relaxation was determined by the addition of acetylcholine (ACh) in an organ-bath system in aortas from septic and sham-operated animals with or without AM/AMBP-1 treatment. The gene and protein expression of ecNOS in aortic and pulmonary tissues was determined by RT-PCR and immunohistochemical analysis, respectively, in those animals with sepsis.
MATERIALS AND METHODS
Experimental animals
Male adult Sprague-Dawley rats (275–315 g), purchased from Charles River Laboratories (Wilmington, MA, USA), were used in this study. All surgical procedures were performed using aseptic technique with the exception of induction of sepsis by cecal ligation and puncture (CLP). The experiments described below were performed in adherence to the National Institutes of Health (NIH) guidelines for the use of experimental animals. This project was approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research.
Animal model of sepsis
Polymicrobial sepsis was induced in rats by CLP as described previously (35). Briefly, rats were fasted overnight prior to the induction of sepsis, but allowed water ad libitum. The animals were anesthetized with isoflurane inhalation, and a 2-cm ventral midline abdominal incision was made. The cecum was then exposed, ligated with 3–0 silk suture just distal to the ileocecal valve to avoid intestinal obstruction, punctured twice with an 18-gauge needle, and returned to the abdominal cavity. The punctured cecum was squeezed to expel a small amount of fecal material. The incision was then closed in layers, and fluid resuscitation by subcutaneous administration of 3 mL/100 g body wt. normal saline was performed immediately after CLP. Sham-operated animals underwent the same surgical procedure except that the cecum was neither ligated nor punctured. Studies were then conducted 20 h after the induction of sepsis or sham-operation. It should be noted that 20 h after CLP represents the late, hypodynamic stage of polymicrobial sepsis (35,36).
Administration of AM/AMBP-1
The fasted animals were anesthetized with isoflurane inhalation, and a 1.0-cm incision was made in the neck. A 200-μL Alzet miniosmotic pump (infusion at a constant rate of 8 μL/h) was prefilled with synthetic rat AM solution (20 μg/ mL sterile normal saline; Pheonix Pharmaceuticals, Belmont, CA, USA) and inserted into the right jugular vein through a silastic catheter. After closure of the neck incision, CLP was performed after the implantation of the pump. Human AMBP-1 (40 μg/kg body wt; Cortex, San Leandro, CA, USA) was then infused via the femoral vein using a Harvard pump at a rate of 50 μL/min for a period of 20 min. The selected dosages of AM and AMBP-1 were previously found to significantly reduce circulating concentrations of TNF-α, IL-1β, and IL-6 (29), and decreased sepsis-induced mortality (30). Vehicle-treated animals received an equal amount of sterile normal saline instead of AM/AMBP-1. Twenty hours after CLP or sham-operation the thoracic aortae and lungs were harvested for various measurements, as described below.
Determination of vascular relaxation
Immediately after the death of the animals by an overdose of isoflurane inhalation, the thoracic cavity was rapidly opened and the thoracic aorta was removed. The blood vessels were immersed in ice-cold Krebs-Ringer bicarbonate solution (composition in mM: NaCl, 118.3; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 25.0; Ca-EDTA, 0.026; glucose, 11.1, Na pyruvate, 1.0) that was aerated with 95% O2: 5% CO2 (pH 7.4; PO2 580 mmHg). The thoracic aorta was cut into approximately 2-mm rings. The aortic rings were then mounted on specimen holders and placed in a glass organ chamber containing 5-mL aerated Krebs-Ringer bicarbonate solution at 37 ºC. One holder was stationary and the other was connected to an isometric force-displacement transducer coupled to a polygraph. The vascular rings were incubated in the aerated Krebs-Ringer bicarbonate solution about 30–60 min. When the basal tension was stable, a contraction of approximately 1-g was induced by 2×10−7 M norepinephrine. An endothelium-dependent vasodilator, acetylcholine (ACh, concentration range from 10−8 to 5×10−6 M) or an endothelium-independent vasodilator, nitroglycerine (NTG, concentration range from 5×10−9 to 10−6 M) was added to the organ bath, and the percentage of vascular relaxation was then determined. At the end of the experiment, the viability of the vascular ring preparations was checked by the addition of norepinephrine. There was no significant decrease in vascular contraction induced by this reagent.
Determination of ecNOS gene expression
The thoracic aortae and lungs (representing blood vessel–rich tissues) were harvested from the sham and septic animals with or without AM/AMBP-1 treatment. Total RNA was extracted and 4 μg RNA was reverse-transcribed. The resulting cDNAs were amplified by PCR reaction using specific primers for rat ecNOS (forward: GGG CCA GGG TGA TGA GCT CTG; reverse: CCC TCC TGG CTT CCA GTG TCC; NM_021838). The PCR reaction was conducted at 35 cycles. Each cycle consisted of 1 min at 94 ºC, 1 min at 65 ºC, and 2 min at 72 ºC. Rat glyceraldehyde 3-phosphate dehydrogenase (G3PDH) served as a housekeeping gene (Clontech). After the RT-PCR procedure, the reaction products were electrophoresed in 1.6% TBE-agarose gel containing 0.22 μg/mL ethidium bromide. The gel was then photographed on Polaroid film and a digital image system was used to determine the band density. The ratio of ecNOS/G3PDH was then calculated.
Determination of ecNOS gene expression in pulmonary tissues using real-time PCR
Total RNA extracted from pulmonary tissues was reverse-transcribed to cDNA as described above. ecNOS gene expression was determined with a real-time PCR technique. The primers specific for rat ecNOS were selected by a real-time PCR primer design software (Primer Express Software Version 3; Applied Biosystems, Foster City, CA, USA) from mRNA sequences retrieved from GenBank (accession #:NM_ 021838; forward primer: CCG GCG CTA CGA AGA ATG; reverse primer: AGT GCC ACG GAT GGA AAT TG). G3PDH was used as a reference gene (accession #M17701; forward primer: ATG ACT CTA CCC ACG GCA AG; reverse primer: CTG GAA GAT GGT GAT GGG TT). Real-time PCR was performed using the 7300 Real-Time PCR system (Applied Biosystems) with SYBR Green as the detection dye. The reaction was carried in a 24 μL final reaction volume containing 0.08 μmol concentrations of each forward and reverse primer, 2 μL cDNA, 9.2 μL H2O and 12 μL SYBR Green PCR Mast Mix (Applied Biosystems). The thermal profile for the real-time PCR was 50 ºC for 2 min and 95 ºC for 10 min, followed by 40 cycles of 95 ºC for 15 s and 60 ºC for 1 min. The gene expression was expressed as fold change according to the formula for real-time PCR data analysis. In addition, melting curve analysis was performed to confirm the specificity of the PCR product in this experiment.
Immunohistochemistry
The aortic tissues were collected 20 h after CLP or sham-operation (n = 4 rats/group) and immediately fixed in the neutralized 4% paraformaldehyde solution overnight. The tissues were then dehydrated and embedded into the paraffin through the standard histology procedure. The paraffin sections were dewaxed and rehydrated, then microwave antigen retrieval was performed. To retrieve the antigen, slides were soaked in 20% citric acid buffer, pH 6.0 (Vector Labs, Burlingame, CA, USA) and kept at 95 ºC for 15 min. The slides were cooled at room temperature for 5 min, rinsed with TBS, then incubated in 3% bovine serum albumin for 30 min to block nonspecific binding followed by incubation in 1:50 rabbit anti-ecNOS polyclonal antibodies (BD Biosciences, San Diego, CA, USA) for two hours at room temperature. After washing, the sections were reacted with 1:200 biotinylated anti-rabbit IgG secondary antibodies (Vector Labs, Burlingame, CA, USA). Vectastain ABC and DAB substrate kits (Vector Labs) were used to reveal the immunohistochemical reaction. Normal rabbit IgG was substituted to primary antibody as a negative control.
Statistical analyses
Data are presented as means ± SE. One-way analysis of variance (ANOVA) and Tukey’s test were used for the comparison among different groups. The difference was considered significant at P ≤ 0.05.
RESULTS
Effects of AM/AMBP-1 on ACh-induced vascular relaxation
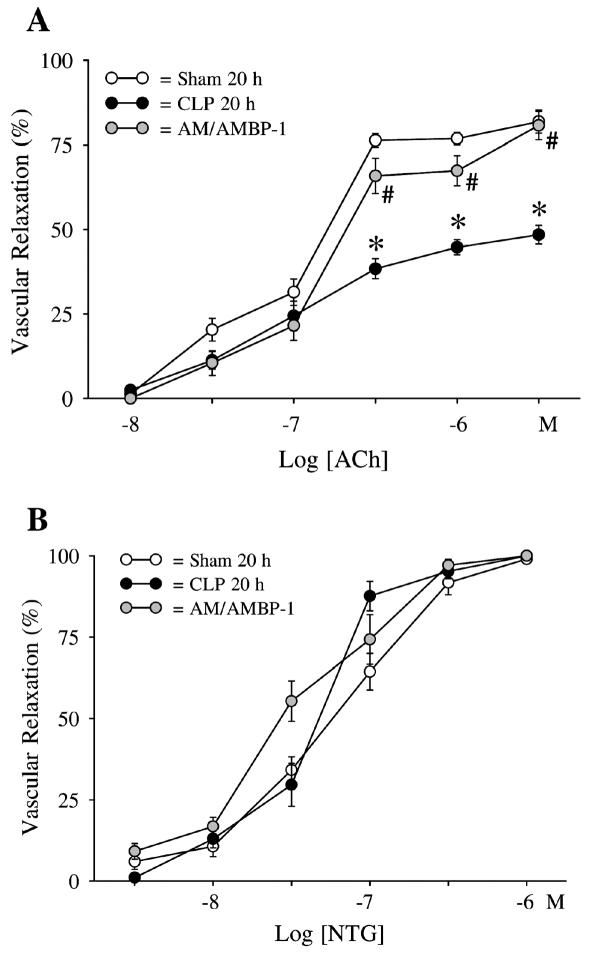
As indicated in Figure 1, vascular relaxation increased to 76–82% at ACh concentrations of 5×10−7 to 5×10−6 M in sham-operated animals. However, ACh-induced vascular relaxation decreased to 38%–48% at the above-mentioned concentrations of ACh in septic animals (P < 0.05). Administration of AM/AMBP-1, however, maintained ACh-induced vascular relaxation to the level similar to sham-operated animals (see Figure 1A). In contrast to ACh, vascular relaxation induced by the endothelium-independent vasodilating agent NTG did not change significantly in septic nor in AM/AMBP-1–treated septic animals compared with sham-operated animals (see Figure 1B). Thus, endothelium-dependent but not endothelium-independent vascular relaxation is reduced in sepsis, and this reduction can be prevented by administration of AM/AMBP-1.
Figure 1.
(A) Effects of AM/AMBP-1 on acetylcholine (ACh)-induced vascular relaxation in aortic rings 20 h after cecal ligation and puncture (CLP). The data are expressed as percentage (%) of the vascular relaxation from the initial tension induced by 2×10−7 M norepinephrine. Values (n = 6/group) are presented as means ± SE and compared by one-way ANOVA and Tukey’s test: *P < 0.05 versus sham-operated animals; #P < 0.05 versus septic animals. (B) Effects of AM/AMBP-1 on nitro-glycerine (NTG)-induced vascular relaxation in the aortic ring 20 h after cecal ligation and puncture (CLP). The data are expressed as percentage (%) of the vascular relaxation from the initial tension induced by 2 ×10−7 M norepinephrine. Values (n = 6/group) are presented as means ± SE. There was no significant difference between sham and septic animals with or without AM/AMBP-1 treatment.
Effects of AM/AMBP-1 on ecNOS gene expression
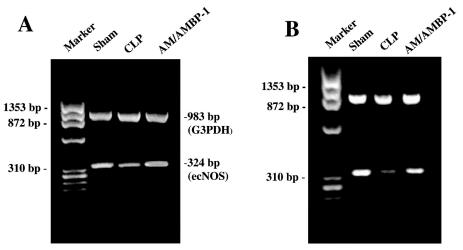
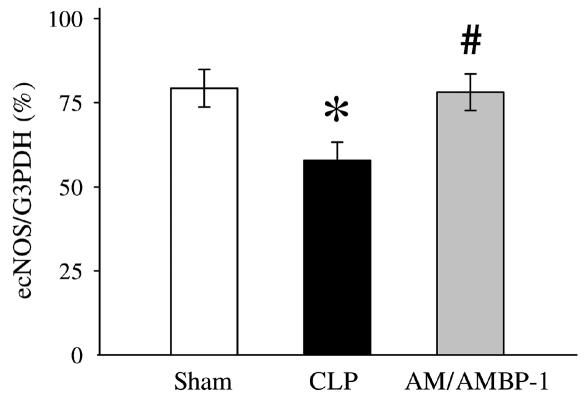
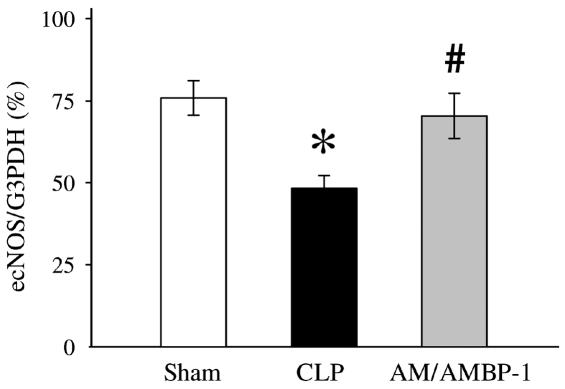
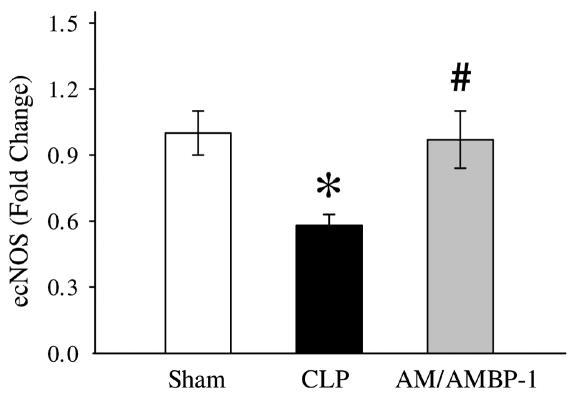
Because ACh-induced vascular relaxation is mediated by NO produced by the activated ecNOS, we decided to determine ecNOS gene expression. In the aortic tissues, ecNOS gene expression decreased by 27% after the onset of sepsis (Figures 2A and 3). However, administration of AM/AMBP-1 prevented the downregulation of ecNOS gene expression in the aortic tissues (Figures 2A and 3). Similarly, pulmonary ecNOS gene expression decreased by 36% in 20 h after CLP, and AM/AMBP-1 treatment maintained its expression to a level similar to that of sham-operated animals (Figures 2B and 4). In addition, real-time PCR results indicate a decrease of 42% in pulmonary ecNOS gene expression at 20 h after CLP, and an increase of 67% in ecNOS gene expression following AM/AMBP-1 treatment (Figure 5).
Figure 2.
Representative gels of the gene expression of ecNOS (324 bp) and housekeeping gene G3PDH (983 bp) in the aortic (A) and pulmonary tissues (B) 20 h after sham-operation or cecal ligation and puncture (CLP) with or without AM/AMBP-1 treatment.
Figure 3.
The ratio of ecNOS versus G3PDH PCR products in the aortic tissues is presented as means ± SE (n = 7/group) and compared by one-way ANOVA and Tukey’s test: *P < 0.05 versus sham-operated animals; #P < 0.05 versus septic animals.
Figure 4.
The ratio of ecNOS versus G3PDH PCR products in the pulmonary tissues is presented as means ± SE (n = 5/group) and compared by one-way ANOVA and Tukey’s test: *P < 0.05 versus sham-operated animals; #P < 0.05 versus septic animals.
Figure 5.
Alteration in pulmonary ecNOS gene expression at 20 h after cecal ligation and puncture (CLP) or sham-operation, measured by real-time PCR technique. The data (fold change) are presented as means ± SE (n = 5) and compared by one-way ANOVA and Tukey’s test. *P < 0.05 versus sham-operated animals; #P < 0.05 versus septic animals.
Effects of AM/AMBP-1 on ecNOS protein levels
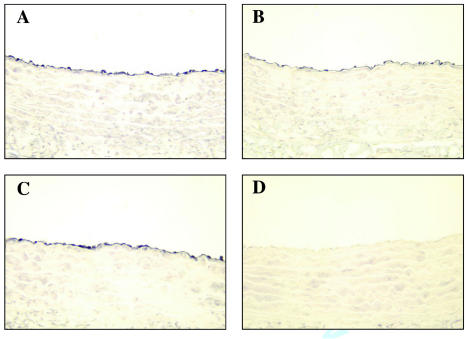
Immunohistochemistry was used to determine the effect of AM/ AMBP-1 on ecNOS protein levels in the aorta. As indicated in Figure 6, ecNOS-positive staining is limited on the top layer of the aortic tissues, indicating aortic endothelial cells (see Figure 6A). At 20 h after the onset of sepsis, ecNOS staining decreased (see Figure 6B), and administration of AM/AMBP-1 prevented such a decrease in ecNOS protein (see Figure 6C).
Figure 6.
The immunohistochemical stainings of ecNOS in the thoracic aortae 20 h after cecal ligation puncture (CLP) with or without AM/AMBP-1 treatment. ecNOS immunoreaction products were observed in aortic endothelial cells (A–C). No immunostaining was seen in vascular smooth muscle or connective tissues. ecNOS immunohistochemical staining in the vascular endothelial cells was reduced in septic animals (B) compared with sham-operated animals (A). Administration of AM/AMBP-1 attenuated the decreased ecNOS staining (C). There were no immunoreaction products in the negative control section (D). (A), sham 20 h; (B), CLP 20 h; (C), CLP 20 h plus AM/AMBP-1 treatment; (D), negative control.
DISCUSSION
We have previously demonstrated that AMBP-1 improves AM-induced vascular relaxation in aortic tissues from septic animals and that vascular levels of AMBP-1 decreases significantly during the late stage of sepsis (28). Our results suggested that AMBP-1 plays an important role in modulating vascular responsiveness to AM during sepsis and circulating AM-AMBP-1 complexes should be considered as an important factor in the transition from hyperdynamic to hypo-dynamic circulation during the progression of polymicrobial sepsis (28). In the present study, we investigated whether AM/AMBP-1 preserves endothelium-dependent vascular relaxation by preventing ecNOS downregulation in sepsis.
Plasma levels of AM begin to rise as early as two hours after CLP and progressively increases to 30 h after the onset of sepsis (37). Elevated levels of AM play a major role in producing the hyperdynamic phase of sepsis, but transition to the late, hypodynamic phase occurs despite continued high circulating levels of AM. Decreased vascular responsiveness to AM appears to explain this paradox and may be responsible for inducing the phase change. Studies of AM-induced vascular relaxation in thoracic aortic rings showed no change at either 5 or 10 h after CLP, but a significant decrease occurred at 20 h after the onset of sepsis (38). These results therefore suggest that vascular AM hyporesponsiveness contributes to the transition from the early, hyperdynamic phase to the late, hypodynamic phase during the progression of sepsis (39). Further study shows that pentoxifylline (PTX) administered after CLP preserved the vascular response to AM in late sepsis (40). PTX treatment did not alter the increase in blood AM concentration following CLP, but it did decrease the elevated levels of TNF-α, IL-1β and IL-6 seen in the late phase (40). In addition to its well known vasodilatory effects, AM also modulates production of inflammatory cytokines. Kaomi et al. (41) reported that AM inhibited secretion of cytokine-induced neutrophil chemoattractant, belonging to IL-8 superfamily in rat alveolar macrophages stimulated with endotoxin. AM also can suppress IL-1β-induced TNF-α production in Swiss 3T3 cells (42).
The novel specific AM binding protein AMBP-1 was first reported by Elsasser et al. (22). AMBP-1 is present in plasma, and the liver is considered to be the main source (29). Our recent studies have demonstrated that coadministration of AM and AMBP-1 prevents the transition from the hyperdynamic phase to the hypodynamic phase, attenuates tissue injury and hemoconcentration during the late hypodynamic stage of sepsis, and decreases sepsis-induced mortality (30). Neither AM nor AMBP-1 alone was sufficient to achieve these beneficial effects (30). Although our recent studies have indicated that increased levels of plasma TNF-α in septic animals were significantly attenuated after the administration of AM/AMBP-1 (29), it remains unknown whether AM/AMBP-1 directly downregulates the proinflammatory cytokines. Kupffer cells are the main source of proinflammatory cytokines in sepsis (43), and we have shown that AM/ AMBP-1 have a direct downregulatory effect on TNF-α from isolated Kupffer cells, as well as from macrophage cell line RAW 264.7 cells (44).
The potential roles of vasoactive mediators other than AM have been evaluated in the pathophysiology of sepsis. Nitric oxide (NO) is a potent vasodilator produced by several isoforms of the enzyme NO synthase (NOS), including endothelial constitutive NOS (ecNOS) and inducible NOS (iNOS). NO derived from ecNOS has been shown to decrease in the early, hyperdynamic phase of sepsis (33,45). Downregulation of ecNOS contributes to vascular hyporesponsiveness in sepsis (32–34). In the present study, ecNOS gene expression decreased by 27%–36% after the onset of sepsis. Two specific mechanisms are responsible for the vasodilatory effect of AM, a direct effect on vascular smooth muscle cells to increase intracellular cAMP levels by stimulating AM receptors and adenylate cyclase activity (39,46,47) and an indirect effect on vascular endothelial cells by stimulating Ca2+ mobilization to increase endothelium-derived NO release via the activation of ecNOS (39,48). Activation of ecNOS was observed to lead to the activation of the NO-cGMP pathway (49,50). In the present study, we observed that the decreased ACh-induced vascular relaxation (reflecting ecNOS-derived NO) at 20 h after CLP was prevented by intravenous administration of AM/AMBP-1. Moreover, the reduced gene expression of endothelial ecNOS observed at 20 h after CLP was prevented after administration of AM/AMBP-1 in aortic (see Figure 3A) and pulmonary (see Figure 3B) tissues. Furthermore, the results also show that the ecNOS-immunoreactive positive area in endothelial cells was significantly reduced in CLP animals, but was restored to normal levels in AM/AMBP-1-treated animals (see Figure 6). It is interesting to note that the endothelium-independent vasodilating agent NTG was not effective in reducing vascular relaxation in septic animals (see Figure 2). This finding suggests that the NO-cGMP pathway is indeed important in mediating the beneficial effects of AM/AMBP-1 in sepsis.
It should be pointed out that the drug-control group (sham-operated animals with AM/AMBP-1 administration) was not included in this study. In this regard, we have previously demonstrated that administration of AM/AMBP-1 in sham-operated rats did not alter circulating levels of liver enzymes (AST, ALT), lactate, creatinine, or inflammatory cytokines (TNF-α, IL-10, HMGB-1) (51). Because it is unlikely that administration of AM/AMBP-1 in shome rats would change ACh-induced vascular relaxation and ecNOS expression, to save animals resources we did not include the drug-control group in the current study. Our previous study clearly showed that AMBP-1 increases AM-induced vascular relaxation in sham-operated animals in a dose-response manner under in vitro conditions (28). Moreover, in vitro addition of AMBP-1 can completely restore AM hyporesponsiveness in the isolated blood vessels in a dose-response fashion (28). Although plasma levels of AM increase in sepsis (37), the levels of AMBP-1 decreased significantly in the blood and tissues under such conditions (28,52). The AM hyporesponsiveness observed in sepsis may be caused by the reduced AMBP-1 production. In addition, the effect of AM/AMBP-1 in sepsis is physiological because AM/AMBP-1 has no adverse effect on sham-operated rats (51). Regarding the potential effects of AM/AMBP-1 on iNOS expression, it is well recognized that iNOS expression and iNOS-derived NO production increase in severe sepsis. While we have clearly shown that administration of AM/AMBP-1 in sepsis improves ecNOS expression and ACh-induced vascular relaxation (ecNOS-mediated), one would expect that AM/AMBP-1 may have suppressive effects on iNOS under such conditions. However, the precise effect of AM/AMBP-1 on iNOS expression and activity in sepsis remains to be determined.
Studies have shown that vascular endothelial cells play an important role in regulation of tissue perfusion by the release of various vasoactive mediators, such as NO, under normal as well as various adverse circulatory conditions (53,54). Endothelium-derived NO is synthesized from L-arginine by Ca2+-dependent, constitutive NO synthase (48). A number of studies have shown that endothelium-derived NO is depressed following endotoxic shock (54,55) as well as during sepsis (33,53). However, the mechanism responsible for the decreased release of endothelial-derived NO during sepsis remains unknown. In the present study, we have provided a clue to the finding that ecNOS gene expression is depressed during sepsis and the protective effect of AM/AMBP-1 in sepsis is to preserve ecNOS gene expression, thereby preventing endothelial cell dysfunction.
ACKNOWLEDGMENTS
This investigation was supported by National Institutes of Health (NIH) grants R01 GM057468 and R01 HL076179 (P Wang). The authors sincerely thank Zheng F Ba, Dale E Fowler, Shaolong Yang, and David A Ornan for their excellent assistance.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 4.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 5.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–24. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 6.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–60. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 8.Sakata J, Shimokubo T, Kitamura K, Nakamura S, Kangawa K, Matsuo H, Eto T. Molecular cloning and biological activities of rat adrenomedullin, a hypotensive peptide. Biochem Biophys Res Commun. 1993;195:921–7. doi: 10.1006/bbrc.1993.2132. [DOI] [PubMed] [Google Scholar]
- 9.Hirata Y, Mitaka C, Sato K, Nagura T, Tsunoda Y, Amaha K, Marumo F. Increased circulating adrenomedullin, a novel vasodilatory peptide, in sepsis. J Clin Endocrinol Metab. 1996;81:1449–53. doi: 10.1210/jcem.81.4.8636349. [DOI] [PubMed] [Google Scholar]
- 10.Nishio K, Akai Y, Murao Y, Doi N, Ueda S, Tabuse H, Miyamoto S, Dohi K, Minamino N, Shoji H, Kitamura K, Kangawa K, Matsuo H. Increased plasma concentrations of adrenomedullin correlate with relaxation of vascular tone in patients with septic shock. Crit Care Med. 1997;25:953–7. doi: 10.1097/00003246-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Ehlenz K, Koch B, Preuss P, Simon B, Koop I, Lang RE. High levels of circulating adrenomedullin in severe illness: correlation with C-reactive protein and evidence against the adrenal medulla as site of origin. Exp Clin Endocrinol Diabetes. 1997;105:156–62. doi: 10.1055/s-0029-1211745. [DOI] [PubMed] [Google Scholar]
- 12.Ueda S, Nishio K, Minamino N, Kubo A, Akai Y, Kangawa K, Matsuo H, Fujimura Y, Yoshioka A, Masui K, Doi N, Murao Y, Miyamoto S. Increased plasma levels of adrenomedullin in patients with systemic inflammatory response syndrome. Am J Respir Crit Care Med. 1999;160:132–6. doi: 10.1164/ajrccm.160.1.9810006. [DOI] [PubMed] [Google Scholar]
- 13.Fujioka S. Increased plasma concentration of adrenomedullin during and after major surgery. Surg Today. 2001;31:575–9. doi: 10.1007/s005950170089. [DOI] [PubMed] [Google Scholar]
- 14.Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, Eto T, Matsio H. Interleukin-1, tumor necrosis factor and lipopolysaccharide additively stimulate production of adrenomedullin in vascular smooth muscle cells. Biochem Biophys Res Commun. 1995;207:25–32. doi: 10.1006/bbrc.1995.1148. [DOI] [PubMed] [Google Scholar]
- 15.Shoji H, Minamina N, Kangawa K, Matsuo H. Endotoxin markedly elevates plasma concentration and gene transcription of adrenomedullin in rat. Biochem Biophys Res Commun. 1995;215:531–7. doi: 10.1006/bbrc.1995.2497. [DOI] [PubMed] [Google Scholar]
- 16.Ono Y, Kojima M, Okada K, Kangawa K. cDNA cloning of canine adrenomedullin and its gene expression in the heart and blood vessels in endotoxin shock. Shock. 1998;10:243–7. doi: 10.1097/00024382-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Zaks-Zilberman M, Salkowski CA, Elsasser T, Cuttitta F, Vogel SN. Induction of adrenomedullin mRNA and protein by lipopolysaccharide and paclitaxel (taxol) in murine macrophages. Infect Immun. 1998;66:4669–75. doi: 10.1128/iai.66.10.4669-4675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elsasser TH, Sartin JL, Martinez A, Kahl S, Montuenga L, Pio R, Fayer R, Miller MJ, Cuttitta F. Underlying disease stress augments plasma and tissue adrenomedullin (AM) responses to endotoxin: colocalized increases in AM and inducible nitric oxide synthase within pancreatic islets. Endocrinol. 1999;140:5402–11. doi: 10.1210/endo.140.11.7132. [DOI] [PubMed] [Google Scholar]
- 19.Matsui E, Kitamura K, Yoshida M, Kato J, Asada Y, Sumiyoshi A, Eto T. Biosynthesis and secretion of adrenomedullin and proadrenomedullin N-terminal 20 peptide in a rat model of endotoxin shock. Hypertens Res. 2001;24:543–9. doi: 10.1291/hypres.24.543. [DOI] [PubMed] [Google Scholar]
- 20.Shindo T, Kurihara H, Kurihara Y, Morita H, Yazaki Y. Upregulation of endothelin-1 and adrenomedullin gene expression in the mouse endotoxin shock model. J Cardiovasc Pharmacol. 1998;31 (Suppl 1):S541–4. doi: 10.1097/00005344-199800001-00156. [DOI] [PubMed] [Google Scholar]
- 21.Zhou M, Chaudry IH, Wang P. The small intestine is an important source of adrenomedullin release during polymicrobial sepsis. Am J Physiol Regul Integr Comp Physiol. 2001;281:R654–60. doi: 10.1152/ajpregu.2001.281.2.R654. [DOI] [PubMed] [Google Scholar]
- 22.Elsasser TH, Kahl S, Martinez A, Montuenga LM, Pio R, Cuttitta F. Adrenomedullin binding protein in the plasma of multiple species: characterization by radioligand blotting. Endocrinol. 1999;140:4908–11. doi: 10.1210/endo.140.10.7157. [DOI] [PubMed] [Google Scholar]
- 23.Pio R, Martinez A, Unsworth EJ, Kowalak JA, Bengoechea JA, Zipfel PF, Elsasser TH, Cuttitta F. Complement factor H is a serum binding protein for adrenomedullin. The resulting complex modulates the bioactivities of both partners. J Biol Chem. 2001;276:12292–300. doi: 10.1074/jbc.M007822200. [DOI] [PubMed] [Google Scholar]
- 24.Friese MA, Hellwage J, Jokiranta TS, Meri S, Peter HH, Eibel H, Zipfel PF. FHL-1/ reconectin and factor H: two human complement regulators which are encoded by the same gene are differently expressed and regulated. Mol Immunol. 1999;36:809–18. doi: 10.1016/s0161-5890(99)00101-7. [DOI] [PubMed] [Google Scholar]
- 25.Schwaeble W, Zwirner J, Schulz TF, Linke RP, Dierich MP, Weiss EH. Human complement factor H: expression of an additional truncated gene product of 43 kDa in human liver. Eur J Immunol. 1987;17:1485–9. doi: 10.1002/eji.1830171015. [DOI] [PubMed] [Google Scholar]
- 26.Luo W, Vik DP. Regulation of complement factor H in a human liver cell line by interferongamma. Scand J Immunol. 1999;49:487–94. doi: 10.1046/j.1365-3083.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- 27.Schwaeble W, Schwaiger H, Brooimans RA, Barbieri A, Most J, Hirsch-Kauffmann M, Tiefenthaler M, Lappin DF, Daha MR, Whaley K. Human complement factor H. Tissue specificity in the expression of three different mRNA species. Eur J Biochem. 1991;198:399–404. doi: 10.1111/j.1432-1033.1991.tb16028.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhou M, Ba ZF, Chaudry IH, Wang P. Adrenomedullin binding protein-1 modulates vascular responsiveness to adrenomedullin in late sepsis. Am J Physiol Regul Integr Comp Physiol. 2002;283:R553–60. doi: 10.1152/ajpregu.00544.2001. [DOI] [PubMed] [Google Scholar]
- 29.Yang S, Zhou M, Fowler DE, Wang P. Mechanisms of the beneficial effect of adrenomedullin and adrenomedullinbinding protein-1 in sepsis: Down-regulation of proinflammatory cytokines. Crit Care Med. 2002;30:2729–35. doi: 10.1097/00003246-200212000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Yang S, Zhou M, Chaudry IH, Wang P. Novel approach to prevent the transition from the hyperdynamic phase to the hypodynamic phase of sepsis: role of adrenomedullin and adrenomedullin binding protein-1. Ann Surg. 2002;236:625–33. doi: 10.1097/00000658-200211000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou M, Simms HH, Wang P. Adrenomedullin and adrenomedullin binding protein-1 attenuate vascular endothelial cell apoptosis in sepsis. Ann Surg. 2004;240:321–30. doi: 10.1097/01.sla.0000133253.45591.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Mitra A, Poole B, Falk S, Lucia MS, Tayal S, Schrier R. Endothelial nitric oxide synthase-deficient mice exhibit increased susceptibility to endotoxin-induced acute renal failure. Am J Physiol Renal Physiol. 2004;287:F1044–8. doi: 10.1152/ajprenal.00136.2004. [DOI] [PubMed] [Google Scholar]
- 33.Zhou M, Wang P, Chaudry IH. Endothelial nitric oxide synthase is downregulated during hyperdynamic sepsis. Biochim Biophys Acta. 1997;1335:182–90. doi: 10.1016/s0304-4165(96)00139-0. [DOI] [PubMed] [Google Scholar]
- 34.Wang P, Ba ZF, Chaudry IH. Nitric oxide: to block or enhance its production during sepsis? Arch Surg. 1994;129:1137–43. doi: 10.1001/archsurg.1994.01420350035003. [DOI] [PubMed] [Google Scholar]
- 35.Wang P, Chaudry IH. A single hit model of polymicrobial sepsis: cecal ligation and puncture. Sepsis. 1998;2:227–33. [Google Scholar]
- 36.Yang S, Cioffi WG, Bland KI, Chaudry IH, Wang P. Differential alterations in systemic and regional oxygen delivery and consumption during the early and late stages of sepsis. J Trauma. 1999;47:706–12. doi: 10.1097/00005373-199910000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Zhou M, Ba Z, Cioffi WG, Chaudry IH. Upregulation of a novel potent vasodilatory peptide adrenomedullin during polymicrobial sepsis. Shock. 1998;10:118–23. doi: 10.1097/00024382-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Wang P, Yoo P, Zhou M, Cioffi WG, Ba ZF, Chaudry IH. Reduction in vascular responsiveness to adrenomedullin during sepsis. J Surg Res. 1999;85:59–66. doi: 10.1006/jsre.1999.5634. [DOI] [PubMed] [Google Scholar]
- 39.Koo DJ, Zhou M, Chaudry IH, Wang P. The role of adrenomedullin in producing differential hemodynamic responses during sepsis. J Surg Res. 2001;95:207–18. doi: 10.1006/jsre.2000.6013. [DOI] [PubMed] [Google Scholar]
- 40.Koo DJ, Yoo P, Cioffi WG, Bland KI, Chaudry IH, Wang P. Mechanism of beneficial effects of pentoxifylline during sepsis: maintenance of adrenomedullin responsiveness and downregulation of proinflammatory cytokines. J Surg Res. 2000;91:70–5. doi: 10.1006/jsre.2000.5916. [DOI] [PubMed] [Google Scholar]
- 41.Kaomi H, Kanazawa H, Hirata K, Kurihara N, Yano Y, Otano S. Adrenomedullin inhibits the secretion of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family, from rat alveolar macrophages. Biochem Biophys Res Commun. 1995;211:1031–5. doi: 10.1006/bbrc.1995.1914. [DOI] [PubMed] [Google Scholar]
- 42.Isumi Y, Kubo A, Katafuchi T, Kangawa K, Minamir N. Adrenomedullin suppresses interleukin-1_-induced tumor necrosis factor-α production in Swiss 3T3 cells. FEBS Lett. 1999;463:110–6. doi: 10.1016/s0014-5793(99)01615-4. [DOI] [PubMed] [Google Scholar]
- 43.Koo DJ, Chaudry IH, Wang P. Kupffer cells are responsible for producing inflammatory cytokines and hepatocellular dysfunction during early sepsis. J Surg Res. 1999;83:151–7. doi: 10.1006/jsre.1999.5584. [DOI] [PubMed] [Google Scholar]
- 44.Wu R, Zhou M, Wang P. Adrenomedullin and adrenomedullin binding protein-1 downregulate TNF-alpha in macrophage cell line and rat Kupffer cells. Regulatory Pept. 2003;112:19–26. doi: 10.1016/s0167-0115(03)00018-1. [DOI] [PubMed] [Google Scholar]
- 45.Wang P, Ba ZF, Chaudry IH. Endothelium dependent relaxation is depressed at the macro-and microcirculatory levels during sepsis. Am J Physiol. 1995;269:R988–93. doi: 10.1152/ajpregu.1995.269.5.R988. [DOI] [PubMed] [Google Scholar]
- 46.Equchi S, Hirata Y, Iwanski H, Sato K, Watanabe TX, Inui P, Nakajima K, Sakakibara S, Marumo F. Structure activity relationship of adrenomedullin, a novel vasodilatory peptide, in cultured rat vascular smooth muscle cells. Endocrinology. 1994;135:2454–8. doi: 10.1210/endo.135.6.7988431. [DOI] [PubMed] [Google Scholar]
- 47.Montuenga LM, Martinez A, Miller MA, Unsworth EJ, Cuttitta F. Expression of adrenomedullin and its receptor during embryogenesis suggests autocrine or paracrine modes of action. Endocrinology. 1997;138:440–51. doi: 10.1210/endo.138.1.4881. [DOI] [PubMed] [Google Scholar]
- 48.Shimekake Y, Nagata K, Ohta S, Kambayashi Y, Teraoka H, Kitamuoa K, Eto P, Kangawa K, Matsuo H. Adrenomedullin stimulated two signal transduction pathways, cAMP accumulation and Ca2+ mobilization in bovine aortic endothelial cells. J Biol Chem. 1995;270:P4412–7. doi: 10.1074/jbc.270.9.4412. [DOI] [PubMed] [Google Scholar]
- 49.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–67. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 50.Nishimatsu H, Suzuki E, Nagata D, Mooiyama N, Satonaka H, Walsh K, Sata M, Kangawa K, Matsuo H, Gotto A, Kitamura T, Hirata Y. Adrenomedullin induces endothelium dependent vasorelaxation via the phosphatidylinositol 3-Kinase/Akt-dependent pathway in rat aorta. Circ Res. 2001;89:63–70. doi: 10.1161/hh1301.092498. [DOI] [PubMed] [Google Scholar]
- 51.Cui X, Wu R, Zhou M, Dong W, Ulloa L, Yang H, Wang H, Tracey KJ, Simms HH, Wang P. Adrenomedullin and its binding protein attenuate the proinflammatory response after hemorrhage. Crit Care Med. 2005;33:391–8. doi: 10.1097/01.ccm.0000153416.41398.a9. [DOI] [PubMed] [Google Scholar]
- 52.Cui Y, Ji Y, Wu R, Zhou M, Wang P. Adrenomedullin binding protein-1 is downregulated during polymicrobial sepsis in the rat. Int J Mol Med. 2006;17:925–9. [PubMed] [Google Scholar]
- 53.Vallet B. Bench-to-bedside review: Endothelial cell dysfunction in sepsis: a role in organ dysfunction? Crit Care. 2003;7:130–8. doi: 10.1186/cc1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamashita T, Kawashina S, Ohashi Y. Resistance to endotoxin shock in transgenic mice overexpressing nitric oxide synthase. Circulation. 2000;101:931–7. doi: 10.1161/01.cir.101.8.931. [DOI] [PubMed] [Google Scholar]
- 55.Parker JL, Adams HR. Selective inhibition of endothelium-dependent vasodilator capacity by Escherichia coli endotoxemia. Circ Res. 1993;72:539–51. doi: 10.1161/01.res.72.3.539. [DOI] [PubMed] [Google Scholar]