Abstract
Aims
As melatonin has been found to play a role in the mechanisms of cardiovascular regulation, we designed the present study to evaluate whether the evening ingestion of the pineal hormone might interfere with the antihypertensive therapy in hypertensive patients well-controlled by nifedipine monotherapy.
Methods
Forty-seven mild to moderate essential hypertensive outpatients taking nifedipine GITS 30 or 60 mg monotherapy at 08.30 h for at least 3 months, were given placebo or melatonin 5 mg at 22.30 h for 4 weeks according to a double-blind cross-over study. At the end of each treatment period patients underwent a 24 h noninvasive ambulatory blood pressure monitoring (ABPM) during usual working days; sleeping period was scheduled to last from 23.00 to 07.00 h.
Results
The evening administration of melatonin induced an increase of blood pressure and heart rate throughout the 24 h period (ΔSBP = + 6.5 mmHg, P < 0.001; ΔDBP = + 4.9 mmHg, P < 0.01; ΔHR = + 3.9 beats min−1, P < 0.01). The DBP as well as the HR increase were particularly evident during the morning and the afternoon hours.
Conclusions
We hypothesize that competition between melatonin and nifedipine, is able to impair the antihypertensive efficacy of the calcium channel blocker. This suggests caution in uncontrolled use of melatonin in hypertensive patients. As the pineal hormone might interfere with calcium channel blocker therapy, it cannot be considered simply a dietary supplement.
Keywords: ABPM, hypertensives, melatonin, nifedipine
Introduction
Several lines of investigation have implicated the pineal hormone, melatonin, as playing a role in the regulation of arterial blood pressure and heart rate in mammals. Pinealectomy enhances the vascular reactivity to vasoconstrictive agents [1] and causes transient hypertension in rats [2–4], which can be reversed by melatonin [5]. Receptors for melatonin have been found in blood vessels of various arterial beds [6–10] and in the heart [9, 11, 12]. An impaired melatonin secretion seems to be present in adult SHR rats [13], in whom the number of arterial receptors for melatonin is increased, without changes in binding affinity [6], when compared with WKY rats, suggesting a receptorial up-regulation in hypertensives. Melatonin administration has been shown to induce a hypotensive effect in both normotensive [14, 15] and spontaneously hypertensive rats [13, 16]. A lowering of arterial blood pressure has also been reported from an uncontrolled study on essential hypertensive patients [17]. A cross-over, placebo-controlled study evaluating the effects of the evening intake of melatonin by young and healthy normotensives subjects [18] showed a mild hypotensive effect during the whole 24 h period, with a concomitant heart rate lowering during the diurnal hours.
These findings suggest that melatonin might have an additive action with antihypertensive agents but no study has been designed to evaluate its possible interaction with other drugs on humans. As melatonin is widely used, mainly as a self-administered sleep-inducing medication, an excessive hypotensive effect might occur in hypertensive treated patients who take the hormone without strict medical control.
The present study was designed to evaluate whether the evening ingestion of melatonin may potentiate the antihypertensive effect of nifedipine monotherapy in well-controlled hypertensive patients.
Methods
This was a double-blind, randomized, placebo-controlled, cross-over study. Fifty mild to moderate essential hypertensive outpatients aged 38–65 years (28 M and 22 F) gave their informed consent to participate in the study, which was previously approved by the local ethics committee. As melatonin may affect the production of sex steroids, suppressing the midcycle surging in luteinizing-hormone secretion and partially inhibiting the ovulation [19], we included only postmenopausal women.
Secondary hypertension had been previously excluded by careful history and through physical and laboratory examinations including radiological and endocrinologic studies. Patients with end organ damage were excluded.
All the patients had taken nifedipine GITS (GastroIntestinal Therapeutic System) 30 or 60 mg once daily at 08.30 h for at least 3 months and their clinic blood pressure was well controlled by this monotherapy (BP < 140/90 mmHg). This formulation was chosen because of its pharmacokinetics, which avoids a reflex sympathoadrenergic activation in chronically treated patients.
All had a regular sleeping-awake schedule (at least 8 h sleep per night, documented by a 1 week diary), no major sleep complaints and had been administered no medication other than nifedipine for 30 days before the study.
After an initial 4 week washout period with placebo, patients were allocated randomly to be administered placebo or melatonin 5 mg at 22.30 h for 4 weeks; after another 4 week washout period with placebo, patients were crossed to the alternative regimen for a further 4 weeks.
Melatonin was obtained from Sigma Aldrich Inc. (Milan, Italy). It was in an immediate-release formulation and capsules were identical in size, shape and colour to the placebo. The time scheduled for ingesting the capsule was chosen according to the recent literature [20] and to the time habitually scheduled for hypnotic drugs. The subjects’ compliance with the treatment was assessed by counting the residual capsules.
At each visit BP measurements were obtained from each patient (right arm) in the seated position, using a standard mercury sphygmomanometer (Korotkoff I and V) with a cuff of appropriate size. Measurements were taken in the morning, by the same observer, after the subjects had rested 10 min in a quiet room. The average of three consecutive measurements, with at least a 5 min interval between each reading, was recorded.
At the end of each treatment period patients underwent 24 h noninvasive ambulatory blood pressure monitoring (ABPM), performed with a SpaceLabs 90207 monitor (SpaceLabs 90207 Inc., Redmond, Washington, USA). It was fitted to the subjects’ non dominant arm at 08.00 h and was set to take readings every 15 min throughout the 24 h. Patients were instructed to remain motionless each time a reading was being taken. Recordings were performed during usual working days (all patients were employees or housewives), and patients were not allowed to nap, to drink caffeinated beverages or alcohol, or to perform any heavy activity.
During the monitoring days lights were to be turned off at 23.00 h and sleeping period was scheduled to last from 23.00 to 07.00 h. In order to document their adherence to the protocol, patients kept a diary in which the times of nocturnal lights off (for sleep) and of morning awakening were reported.
Blood pressure values from 07.00 to 23.00 h were chosen to determine the daytime level and values from 23.00 to 07.00 were used for nocturnal values.
The nocturnal fall in blood pressure was calculated as the mean percentage dip with respect to daytime values. Twenty-four hour recordings were discarded from the analysis when more than 10% of all readings, or more than one reading per hour, were missing or erroneous.
Data are presented as means ± s.d. Statistical analysis of the data was performed using version 6.04 of the SAS system (SAS Institute Inc., Cary, USA). Analysis of variance and paired Student’s t-tests were used. P < 0.05 was considered statistically significant.
In order to verify the basic assumptions of the cross-over design, besides the evaluation of the period effect, the possible presence of carry-over or sequence effects was also investigated [21]. However, for no variable was a period effect or, more specifically, a sequence effect found.
Results
Forty seven out the fifty enrolled patients completed the study (27 men and 20 women). Two subjects dropped out because they had a 10% or more loss of their ambulatory blood pressure data and they did not consent to repeat the 24 h recordings. One patient dropped out complaining of marked weakness.
At enrolment office blood pressure and heart rate were 136 ± 10/85 ± 8 mmHg and 72 ± 5 beats min−1, respectively; at the end of the active treatment period (melatonin) BP and HR were 138 ± 11/87 ± 8 mmHg and 75 ± 5 beats min−1, and at the end of the placebo period BP and HR were 137 ± 10/85 ± 8 mmHg and 73 ± 5 beats min−1, respectively; at the end of each treatment no statistical differences in office BP and HR were observed respect to the basal values.
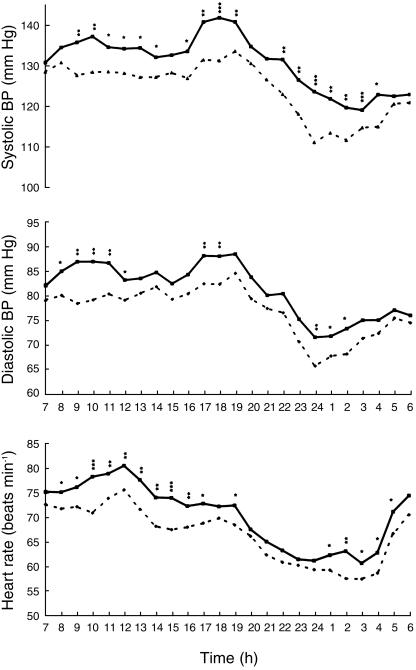
Figure 1 and Table 1 show the mean ambulatory blood pressure and heart rate values recorded during melatonin intake compared with values after placebo.
Figure 1.
Ambulatory blood pressure and heart rate monitorings during melatonin (▪) and placebo (▴) treatment. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 1.
Mean systolic (SBP), diastolic (DBP) and heart rate (HR) ambulatory values during placebo and melatonin intake.
| Placebo | Melatonin | ||
|---|---|---|---|
| SBP (mm Hg) | P | ||
| 24 h | 124.16 ± 9 | 130.65 ± 10 | < 0.001 |
| Daytime | 128.53 ± 10 | 134.91 ± 11 | < 0.002 |
| Nighttime | 115.43 ± 7 | 122.11 ± 8 | < 0.001 |
| DBP (mm Hg) | P | ||
| 24 h | 76.71 ± 5 | 81.0 ± 6 | < 0.01 |
| Daytime | 79.90 ± 7 | 84.51 ± 7 | < 0.002 |
| Nighttime | 70.33 ± 4 | 73.96 ± 5 | < 0.01 |
| HR (beats min−1) | P | ||
| 24 h | 66.50 ± 3 | 70.42 ± 4 | < 0.01 |
| Daytime | 69.23 ± 4 | 73.41 ± 4 | < 0.01 |
| Nighttime | 61.05 ± 3 | 64.43 ± 3 | < 0.05 |
The evening administration of melatonin induced an increase in blood pressure throughout the 24 h period (ΔSBP = + 6.5 mmHg, 95% CI: 2.3, 10.7, P < 0.001; ΔDBP = + 4.9 mmHg, 95% CI: 1.2, 8.4, P < 0.01), without modifying the 24 h BP profile.
The nocturnal fall in SBP and DBP (the degree of average nocturnal fall compared to the daytime average) did not change after administration of melatonin (10.2% vs 9.5% and 12% vs 12.5%, respectively, after melatonin and placebo). The increase of SBP with melatonin was higher during the afternoon (Δ SBP 14.00 h-19.00 h = + 7.1 mmHg, P < 0.001) and the first part of the night (Δ SBP 22.00 h-02.00 h = + 9.5 mmHg, P < 0.001). The increase of DBP was higher during the morning (Δ DBP 08.00–12.00 h = + 6.9 mmHg, P < 0.001).
Melatonin induced an increase in HR throughout the 24 h period (ΔHR = + 3.9 beats min−1, 95% CI: 1,4, 6.4, P < 0.05), being higher during the morning hours (ΔHR 08.00–12.00 h = + 4.5 beats min−1, P < 0.001) and the afternoon (ΔHR 13.00–19.00 h = + 4.8 beats min−1, P < 0.001).
Concerning the side-effects of which patients complained spontaneously, drowsiness during the morning was reported by 15 subjects taking melatonin and by two subjects after placebo (P < 0.01). Ten patients being administered melatonin and three patients taking placebo complained of weakness (P < 0.05).
Discussion
The results obtained in the present study show that the chronic evening ingestion of melatonin in hypertensive patients well controlled by nifedipine GITS induces a blood pressure increase and a heart rate acceleration. The SBP increase is present for almost the whole 24 h period, while the DBP increase, also present through the 24 h, was particularly evident during the morning hours. The heart rate too tended to be higher for the entire 24 h period, although the increase was statistically significant and clinically important only during the morning and the afternoon hours.
As the administration of the exogenous pineal hormone had previously been shown to lower blood pressure and heart rate either in young normotensive subjects [18] or in hypertensive patients [17], as well as in SHR rats [3, 16], we expected to observe an additive effect between melatonin and nifedipine in hypertensive patients. The completely different results obtained suggest a possible kinetic or pharmacodynamic interaction between melatonin and nifedipine with an effect which impairs the antihypertensive efficacy of the calcium channel blocker.
Many studies in vitro and in vivo on the relationship between melatonin and calcium channels [22–26] suggest that the hormone might directly affect calcium signalling by interacting with calmodulin [27] or target enzymes such as adenylate cyclase and phospodiesterase, as well as with structural proteins [28, 29]. Satake et al. [30] found that nifedipine and verapamil inhibit the melatonin-induced relaxation of aorta precontracted with 5-HT in vitro, but this would explain an inhibition of melatonin vasodilation by nifedipine and not the reverse.
The increase in BP and HR observed after melatonin administration might be due to an increased sensitivity of arteries to noradrenaline [31–33], or to an increase of the sensitivity to the baroreflex elicited by nifedipine. Although nifedipine GITS, which is a slow release form of nifedipine, does not change HR even under acute conditions, it elicits a marked activation of peripheral muscle sympathetic nerve activity, measured in the peroneal nerve [34].
Laflamme et al. [16] found that when SHR are pretreated with melatonin, the sympathoadrenal reflex reactivity to nitroprusside is potentiated, with a 0.7- and a 1.7-fold increase in noradrenaline and adrenaline levels, respectively. The same mechanism might play a role with nifedipine.
At the moment we cannot give a clear explanation of our results. Further studies are needed, including the evaluation of sympathetic nerve activity, and metabolism and disposition of nifedipine and melatonin, in order to best understand the possible interactions of the two drugs. However this preliminary study has clinical implications. Even if the clinic BP and HR increase observed while taking melatonin are not statistically significant, the BP load, defined as the percentage of abnormal BP readings recorded by ABPM (SBP > 140 mmHg and DBP > 90 mmHg) in a 24 h period [43, 44], was significantly increased by melatonin. In hypertension chronic overload induces myocardial and vascular damage and there are data to support the view that BP load is a better determinant of cardiac and vascular abnormalities than casual BP values [45, 46].
Melatonin users are the unwitting subjects in a large-scale uncontrolled experiments [35], although the only recommended medical use is the short-term management of jet-lag symptoms [36–42], being the only beneficial effect of melatonin that has been verified by well-controlled trials with sufficiently large samples. The results of the present study suggest caution in uncontrolled use of melatonin in hypertensive patients. As the pineal hormone might interfere with calcium channel blocker therapy, it cannot be considered simply a dietary supplement, and its use should be restricted to its proven medical indication.
References
- 1.Cunnane SC, Manku MS, Oka M, Horrobin DF. Enhanced vascular reactivity to various agents following pinealectomy in the rats: role of melatonin. Can J Physiol Pharmacol. 1980;58:287–293. doi: 10.1139/y80-049. [DOI] [PubMed] [Google Scholar]
- 2.Zanoboni A, Forni A, Zanobini-muciaccia W, Zanussi C. Effect of pinealectomy on arterial blood pressure and food and water intake in the rat. J Endocrinol Invest. 1978;2:125–130. doi: 10.1007/BF03350359. [DOI] [PubMed] [Google Scholar]
- 3.Zanoboni A, Zanobini-muciaccia W. Experimental hypertension in pinealectomized rats. Life Sci. 1967;6:2327–2331. doi: 10.1016/0024-3205(67)90043-4. [DOI] [PubMed] [Google Scholar]
- 4.Karppanen H, Vapatalo H, Lahovara S, Pasonen MK. Pinealectomy-induced hypertension in the rat. Acta Physiol Scand Supplement. 1969;330:94–101. [Google Scholar]
- 5.Holmes SV, Sudgen D. The effect of melatonin on pinealectomy-induced hypertension in the rat. Br J Pharmacol. 1976;56:360–364. [PMC free article] [PubMed] [Google Scholar]
- 6.Viswanathan M, Laitinen JT, Saavedra JM. Differential expression of melatonin receptors in spontaneously hypertensive rats. Neuroendocrinology. 1992;56:864–870. doi: 10.1159/000126318. [DOI] [PubMed] [Google Scholar]
- 7.Krause DN, Barrios VE, Duckles SP. Melatonin receptors mediate potentiation of contractile responses to adrenergic nerve stimulation in rat caudal artery. Eur J Pharmacol. 1995;276:207–213. doi: 10.1016/0014-2999(95)00028-j. [DOI] [PubMed] [Google Scholar]
- 8.Viswanathan H, Laitinen JT, Saavedra JM. Expression of melatonin receptors in arteries involved in thermoregulation. Proc Natl Acad Sci USA. 1990;87:6200–6203. doi: 10.1073/pnas.87.16.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pang SF, Dubocovichj ML, Brown GM. Melatonin receptors in peripheral tissue: a new area of melatonin research. Biol Signals. 1993;2:177–180. doi: 10.1159/000109490. [DOI] [PubMed] [Google Scholar]
- 10.Capsoni S, Viswanathan M, De Oliveira AM, Saavedra JM. Characterization of melatonin receptors and signal transduction system in rat arteries forming the Circle of Willis. Endocrinology. 1994;135:373–378. doi: 10.1210/endo.135.1.8013371. [DOI] [PubMed] [Google Scholar]
- 11.Pang CS, Brown GM, Tang PL, Cheng KM, Pang SF. 2-[125 I]iodomelatonin binding sites in the lung and heart: a link between the photoperiodic signal, melatonin, and the cardiopulmonary system. Biol Signals. 1993;2:228–236. doi: 10.1159/000109496. [DOI] [PubMed] [Google Scholar]
- 12.Pang CS, Tang PL, Song Y, Brown GM, Pang SF. 2-[125 I]iodomelatonin binding sites in the quail heart: characteristics, distribution and modulation by guanine nucleotides and cations. Life Sci. 1996;58:1047–1057. doi: 10.1016/0024-3205(96)00058-6. [DOI] [PubMed] [Google Scholar]
- 13.Kawashima K, Miwa Y, Fujimoto K, Oohata H, Nishino H, Koike H. Antihypertensive action of melatonin in the spontaneously hypertensive rats. Clin Exp Hypertens. 1987;9:1121–1131. doi: 10.3109/10641968709160037. [DOI] [PubMed] [Google Scholar]
- 14.Harlow HJ. Influence of the pineal gland and melatonin on blood flow and evaporative water loss during heat stress in rats. J Pineal Res. 1987;4:147–159. doi: 10.1111/j.1600-079x.1987.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 15.Chuang J, Chen SS, Lin MT. Melatonin decreases brain serotonin release, arterial pressure and heart rate in rats. Pharmacology. 1993;47:91–97. doi: 10.1159/000139083. [DOI] [PubMed] [Google Scholar]
- 16.Laflamme AK, Wu L, Foucart S, de Champlain J. Impaired basal sympathetic tone and α1-adrenergic responsiveness in association with the hypotensive effect of melatonin in spontaneously hypertensive rats. Am J Hypertens. 1998;11:219–229. doi: 10.1016/s0895-7061(97)00401-9. [DOI] [PubMed] [Google Scholar]
- 17.Birau N, Peterssen U, Meyer C, Gottschalck J. Hypotensive effect of melatonin in essential hypertension. IRCS Med Sci. 1981;9:906. [Google Scholar]
- 18.Lusardi P, Preti P, Savino S, Piazza E, Zoppi A, Fogari R. Effect of bedtime melatonin ingestion on blood pressure of normotensive subjects. Blood Pressure Monitoring. 1997;2:99–103. [PubMed] [Google Scholar]
- 19.Voordouw BCG, Euser R, Verdonk RER, et al. Melatonin and melatonin-progestin combinations alter pituitary-ovarian function in women and can inhibit ovulation. J Clin Endocrinol Metab. 1992;74:108–117. doi: 10.1210/jcem.74.1.1727807. [DOI] [PubMed] [Google Scholar]
- 20.Wurtman RJ, Zhdanova I. Improvement of sleep quality by melatonin. Lancet. 1995;346:1491. doi: 10.1016/s0140-6736(95)92509-0. [DOI] [PubMed] [Google Scholar]
- 21.Senn SL. Cross-over trials in clinical research. New York: John Wiley & Sons; 1993. [Google Scholar]
- 22.Chen LD, Kumar P, Reiter RJ, Tan DX, Chamber JP, Manchester LC, Poeggeler B. Melatonin reduces 3H-nitrendipine binding in the heart. Proc Soc Exp Biol Med. 1994;207:34–37. doi: 10.3181/00379727-207-43787. [DOI] [PubMed] [Google Scholar]
- 23.Uchida K, Aoki T, Satoh H, Tajiri O. Effects of melatonin on muscle contractility and neuromuscolar blockade produced by muscle relaxants. Masui. 1997;46:205–212. [PubMed] [Google Scholar]
- 24.Muck AO, Seeger H, Bartsch C, Lippert TH. Does melatonin affect calcium influx in human aortic smooth cells and estradiol-mediated calcium antagonism ? J Pineal Res. 1996;20:145–147. doi: 10.1111/j.1600-079x.1996.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 25.Benitez-king G, Huerto-delgadillo L, Anton-tay F. Melatonin modifies calmodulin cell levels in MDCK and N1E-115 cell lines and inhibits phosphodiesterase activity in vitro. Brain Res. 1991;557:289–292. doi: 10.1016/0006-8993(91)90146-m. [DOI] [PubMed] [Google Scholar]
- 26.Benitez-king G, Huerto-delgadillo L, Anton-tay F. Binding of 3H-melatonin to calmodulin. Life Sci. 1993;53:201–207. doi: 10.1016/0024-3205(93)90670-x. [DOI] [PubMed] [Google Scholar]
- 27.Benitez-king G, Rios A, Martinez A, Anton-tay F. In vitro inhibition of Ca2+/calmodulin-dependent kinase II activity by melatonin. Biochim Biophys Acta. 1996;1290:191–196. doi: 10.1016/0304-4165(96)00025-6. [DOI] [PubMed] [Google Scholar]
- 28.Benitez-king G, Anton-tay F. Calmodulin mediates melatonin cytoskeletal effects. Experientia. 1993;49:635–641. doi: 10.1007/BF01923944. [DOI] [PubMed] [Google Scholar]
- 29.Godson C, Reppert SM. The Mel1a melatonin receptor is coupled to parallel signal transduction pathways. Endocrinology. 1997;138:397–404. doi: 10.1210/endo.138.1.4824. [DOI] [PubMed] [Google Scholar]
- 30.Satake N, Oe H, Shibata S. Vasorelaxing action of melatonin in rat isolated aorta; possible endothelium dependent relaxation. Gen Pharmacol. 1991;22:1127–1133. doi: 10.1016/0306-3623(91)90589-x. [DOI] [PubMed] [Google Scholar]
- 31.Weekley LB. Effects of melatonin on pulmonary and coronary vessels are exerted through perivascular nerves. Clin Auton Res. 1993;3:45–47. doi: 10.1007/BF01819143. [DOI] [PubMed] [Google Scholar]
- 32.Evans BK, Mason R, Wilson VG. Evidence for vasoconstrictor activity of melatonin in ‘pressurized’ segments of isolated caudal artery from juvenile rats. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:362–365. doi: 10.1007/BF00173553. [DOI] [PubMed] [Google Scholar]
- 33.Duckles SP, Barrios VE, Doolen S, Krause D. Melatonin receptors potentiate contractile responses to adrenergic nerve stimulation in rat caudal artery. Proc West Pharmacol Soc. 1995;38:101–102. [PubMed] [Google Scholar]
- 34.Wenzel WW, Allegranza G, Binggeli C, et al. Differential activation of cardiac and peripheral sympathetic nervous system by nifedipine: role of pharmacokinetics. J Am Coll Cardiol. 1997;29:1607–1614. doi: 10.1016/s0735-1097(97)00095-8. [DOI] [PubMed] [Google Scholar]
- 35.Lamberg L. Melatonin potentially useful but safety, efficacy remain uncertain. JAMA. 1996;276:1011–1014. [PubMed] [Google Scholar]
- 36.Arendt J, Aldhous M, English J, Marks V, Arendt JH, Marks M, et al. Some effects of jet-lag and their alleviation by melatonin. Ergonomics. 1987;30:1379–1393. [Google Scholar]
- 37.Petrie K, Dawson AG, Thompson L, Brook R. A double blind trial of melatonin as a treatment for jet-lag in international cabin crew. Biol Psychiatry. 1993;33:526–530. doi: 10.1016/0006-3223(93)90007-z. [DOI] [PubMed] [Google Scholar]
- 38.Claustrat B, Brun J, Sassolas G, Charzot G. Melatonin and jet-lag: confirmatory result using a simplified protocol. Biol Psychiatry. 1993;34:587. doi: 10.1016/0006-3223(92)90300-o. [DOI] [PubMed] [Google Scholar]
- 39.Travel statement of jet-lag. Committee to Advise on Tropical Medicine and Travel (CATMAT) Can Commun Dis Rep. 1995;21:148–151. [PubMed] [Google Scholar]
- 40.Arendt J. Melatonin and the Mammalian Pineal Gland. Chapman & Hall; 1995. Jet-lag and shift work; pp. 263–265. [Google Scholar]
- 41.Comperatore CA, Lieberman HR, Kirby AW, Adams B, Crowley JS. Melatonin efficacy in aviation missions requiring rapid deployment and night operations. Aviat Space Environ Med. 1996;67:520–524. [PubMed] [Google Scholar]
- 42.Brzezinsky A. Melatonin in humans. New Engl J Med. 1997;16:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 43.Zacharias PK, Sheps SG, Baily KR, Wiltgen CM, More AG. Age-related characteristics of ambulatory blood pressure load and mean blood pressure in normotensive subjects. JAMA. 1991;265:1414–1417. [PubMed] [Google Scholar]
- 44.Zacharias PK, Sheps SG, Ilstrup DM, et al. Blood pressure load, a better determinant of hypertension. Mayo Clin Proc. 1988;63:1085–1091. doi: 10.1016/s0025-6196(12)65503-7. [DOI] [PubMed] [Google Scholar]
- 45.White WB. Blood pressure load and target organ effects in patients with essential hypertension. J Hypertens. 1991;9(Suppl 8):S39–S41. [PubMed] [Google Scholar]
- 46.Verdecchia P, Schillaci G, Guerrini M, et al. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1991;81:528–536. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]