Abstract
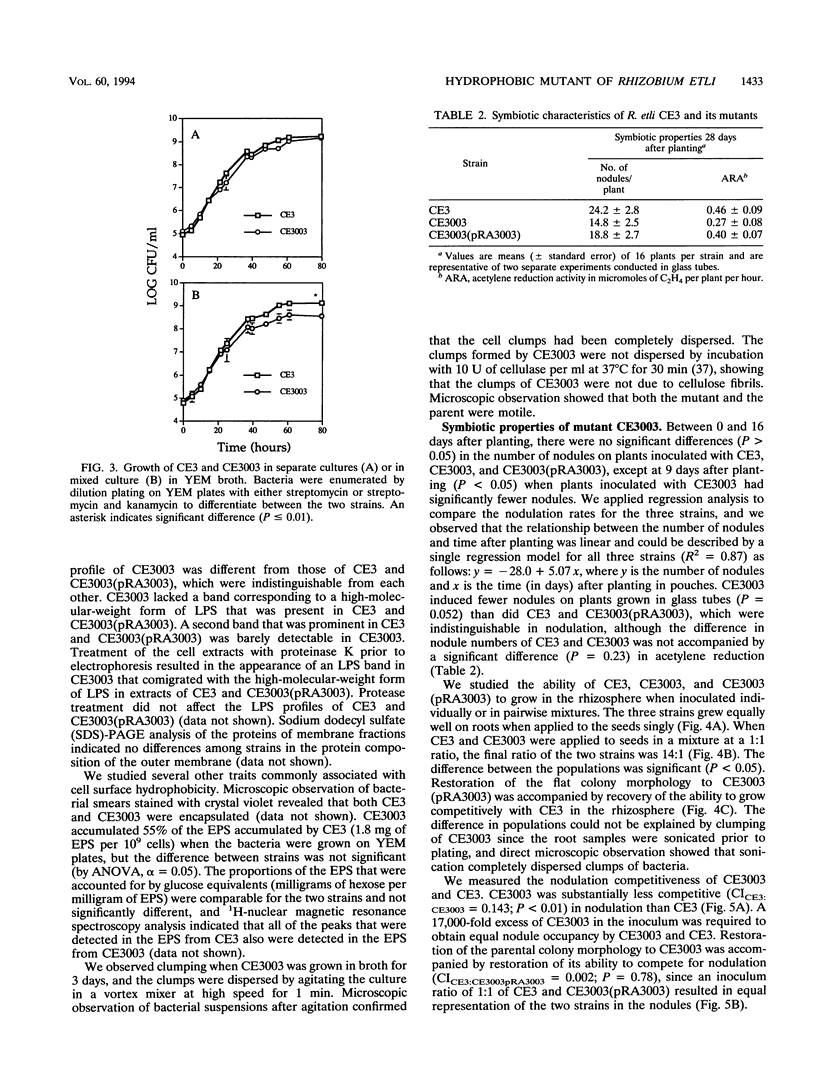
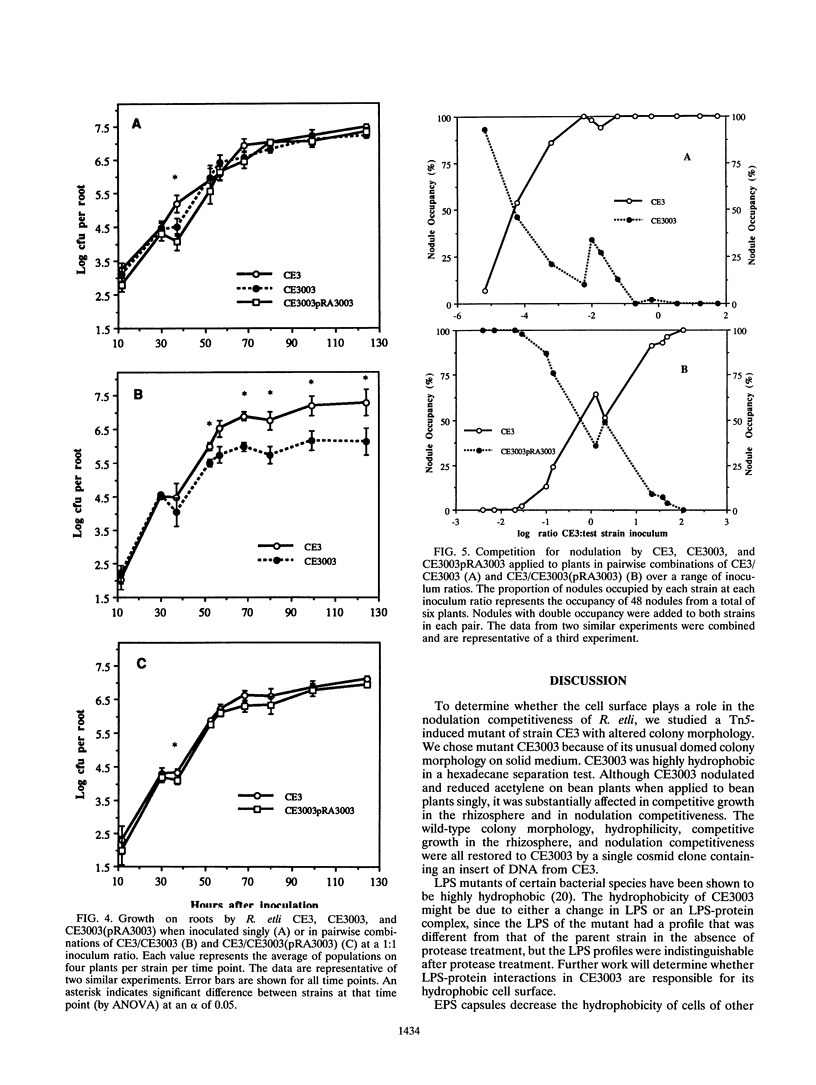
We isolated and characterized CE3003, a Tn5-induced mutant with altered colony morphology derived from Rhizobium etli CE3. CE3003 produced domed colonies and was highly hydrophobic as indicated by its ability to partition into hexadecane, whereas its parent produced flat colonies and was hydrophilic. On bean plants, CE3003 induced nodules and reduced acetylene. CE3003 and CE3 grew at similar rates when they were grown separately or together in culture medium or inoculated singly onto bean seeds. However, when they were mixed at a 1:1 ratio and applied to seeds, CE3003 achieved significantly lower populations than CE3 in the rhizosphere. Five days after coinoculation of CE3 and CE3003, the population of the mutant was less than 10% of the population of CE3 in the bean rhizosphere. To determine the nodulation competitiveness of the mutant, it was coinoculated with CE3 at various ratios at planting, and the ratio of the nodules occupied by each strain was determined 21 days later. A 17,000-fold excess of CE3003 in mixed inocula was required to obtain equal nodule occupancy by the two strains. A genomic library of strain CE3 was mobilized into CE3003, and we identified a cosmid, pRA3003, that restored the parental colony morphology and hydrophilicity to the mutant. Restoration of the parental colony morphology was accompanied by recovery of the ability to grow competitively in the rhizosphere and to compete for nodulation of beans. The data show an association between cell surface hydrophobicity, nodulation competitiveness, and competitive growth in the rhizosphere in mutant CE3003.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. N., Hanson R. S. Construction of broad-host-range cosmid cloning vectors: identification of genes necessary for growth of Methylobacterium organophilum on methanol. J Bacteriol. 1985 Mar;161(3):955–962. doi: 10.1128/jb.161.3.955-962.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo R. S., Maya-Flores J., Barnes-McConnell D., Yokoyama C., Dazzo F. B., Bliss F. A. Semienclosed Tube Cultures of Bean Plants (Phaseolus vulgaris L.) for Enumeration of Rhizobium phaseoli by the Most-Probable-Number Technique. Appl Environ Microbiol. 1986 Oct;52(4):954–956. doi: 10.1128/aem.52.4.954-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti L., Lara J. C., Leigh J. A. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5625–5629. doi: 10.1073/pnas.89.12.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie G. A., Clayton M. K., Handelsman J. Quantitative comparison of the laboratory and field competitiveness of Rhizobium leguminosarum biovar phaseoli. Appl Environ Microbiol. 1989 Nov;55(11):2755–2761. doi: 10.1128/aem.55.11.2755-2761.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie G. A., Handelsman J. Evaluation of a strategy for identifying nodulation competitiveness genes in Rhizobium leguminosarum biovar phaseoli. J Gen Microbiol. 1993 Mar;139(3):529–538. doi: 10.1099/00221287-139-3-529. [DOI] [PubMed] [Google Scholar]
- Benedí V. J., Ciurana B., Tomás J. M. Isolation and characterization of Klebsiella pneumoniae unencapsulated mutants. J Clin Microbiol. 1989 Jan;27(1):82–87. doi: 10.1128/jcm.27.1.82-87.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Bhagwat A. A., Keister D. L. Identification and cloning of Bradyrhizobium japonicum genes expressed strain selectively in soil and rhizosphere. Appl Environ Microbiol. 1992 May;58(5):1490–1495. doi: 10.1128/aem.58.5.1490-1495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwat A. A., Tully R. E., Keister D. L. Isolation and Characterization of a Competition-Defective Bradyrhizobium japonicum Mutant. Appl Environ Microbiol. 1991 Dec;57(12):3496–3501. doi: 10.1128/aem.57.12.3496-3501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwat A. A., Tully R. E., Keister D. L. Isolation and characterization of an ndvB locus from Rhizobium fredii. Mol Microbiol. 1992 Aug;6(15):2159–2165. doi: 10.1111/j.1365-2958.1992.tb01389.x. [DOI] [PubMed] [Google Scholar]
- Brewin N. J. Development of the legume root nodule. Annu Rev Cell Biol. 1991;7:191–226. doi: 10.1146/annurev.cb.07.110191.001203. [DOI] [PubMed] [Google Scholar]
- Cava J. R., Elias P. M., Turowski D. A., Noel K. D. Rhizobium leguminosarum CFN42 genetic regions encoding lipopolysaccharide structures essential for complete nodule development on bean plants. J Bacteriol. 1989 Jan;171(1):8–15. doi: 10.1128/jb.171.1.8-15.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty A. K., Zurkowski W., Shine J., Rolfe B. G. Symbiotic nitrogen fixation: molecular cloning of Rhizobium genes involved in exopolysaccharide synthesis and effective nodulation. J Mol Appl Genet. 1982;1(6):585–596. [PubMed] [Google Scholar]
- Cook D., Sequeira L. Genetic and biochemical characterization of a Pseudomonas solanacearum gene cluster required for extracellular polysaccharide production and for virulence. J Bacteriol. 1991 Mar;173(5):1654–1662. doi: 10.1128/jb.173.5.1654-1662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold R., Noel K. D. Rhizobium leguminosarum exopolysaccharide mutants: biochemical and genetic analyses and symbiotic behavior on three hosts. J Bacteriol. 1989 Sep;171(9):4821–4830. doi: 10.1128/jb.171.9.4821-4830.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic S. P., Chen H., Batley M., Redmond J. W., Rolfe B. G. Nitrogen fixation ability of exopolysaccharide synthesis mutants of Rhizobium sp. strain NGR234 and Rhizobium trifolii is restored by the addition of homologous exopolysaccharides. J Bacteriol. 1987 Jan;169(1):53–60. doi: 10.1128/jb.169.1.53-60.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dénarié J., Debellé F., Rosenberg C. Signaling and host range variation in nodulation. Annu Rev Microbiol. 1992;46:497–531. doi: 10.1146/annurev.mi.46.100192.002433. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Signal exchange in plant-microbe interactions. Microbiol Rev. 1986 Jun;50(2):193–225. doi: 10.1128/mr.50.2.193-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Handelsman J., Ugalde R. A., Brill W. J. Rhizobium meliloti competitiveness and the alfalfa agglutinin. J Bacteriol. 1984 Mar;157(3):703–707. doi: 10.1128/jb.157.3.703-707.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kamicker B. J., Brill W. J. Identification of Bradyrhizobium japonicum Nodule Isolates from Wisconsin Soybean Farms. Appl Environ Microbiol. 1986 Mar;51(3):487–492. doi: 10.1128/aem.51.3.487-492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Lagares A., Caetano-Anollés G., Niehaus K., Lorenzen J., Ljunggren H. D., Pühler A., Favelukes G. A Rhizobium meliloti lipopolysaccharide mutant altered in competitiveness for nodulation of alfalfa. J Bacteriol. 1992 Sep;174(18):5941–5952. doi: 10.1128/jb.174.18.5941-5952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Milner J. L., Araujo R. S., Handelsman J. Molecular and symbiotic characterization of exopolysaccharide-deficient mutants of Rhizobium tropici strain CIAT899. Mol Microbiol. 1992 Nov;6(21):3137–3147. doi: 10.1111/j.1365-2958.1992.tb01770.x. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Sanchez A., Fernandez L., Leemans J., Cevallos M. A. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984 Apr;158(1):148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Whitnack E., Beachey E. H. Hydrophobic interactions of group A streptococci with hexadecane droplets. J Bacteriol. 1983 Apr;154(1):139–145. doi: 10.1128/jb.154.1.139-145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia L., Young J. P., Martínez-Romero E. Reclassification of American Rhizobium leguminosarum biovar phaseoli type I strains as Rhizobium etli sp. nov. Int J Syst Bacteriol. 1993 Apr;43(2):374–377. doi: 10.1099/00207713-43-2-374. [DOI] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J Bacteriol. 1983 Dec;156(3):1292–1300. doi: 10.1128/jb.156.3.1292-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W. Isolation of genes involved in nodulation competitiveness from Rhizobium leguminosarum bv. trifolii T24. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3810–3814. doi: 10.1073/pnas.85.11.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R. A., Rao A. S., Mulders I. H., Goosen-de Roo L., van Loosdrecht M. C., Wijffelman C. A., Lugtenberg B. J. Isolation and characterization of mutants of Rhizobium leguminosarum bv. viciae 248 with altered lipopolysaccharides: possible role of surface charge or hydrophobicity in bacterial release from the infection thread. J Bacteriol. 1989 Feb;171(2):1143–1150. doi: 10.1128/jb.171.2.1143-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]




