Abstract
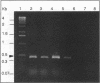
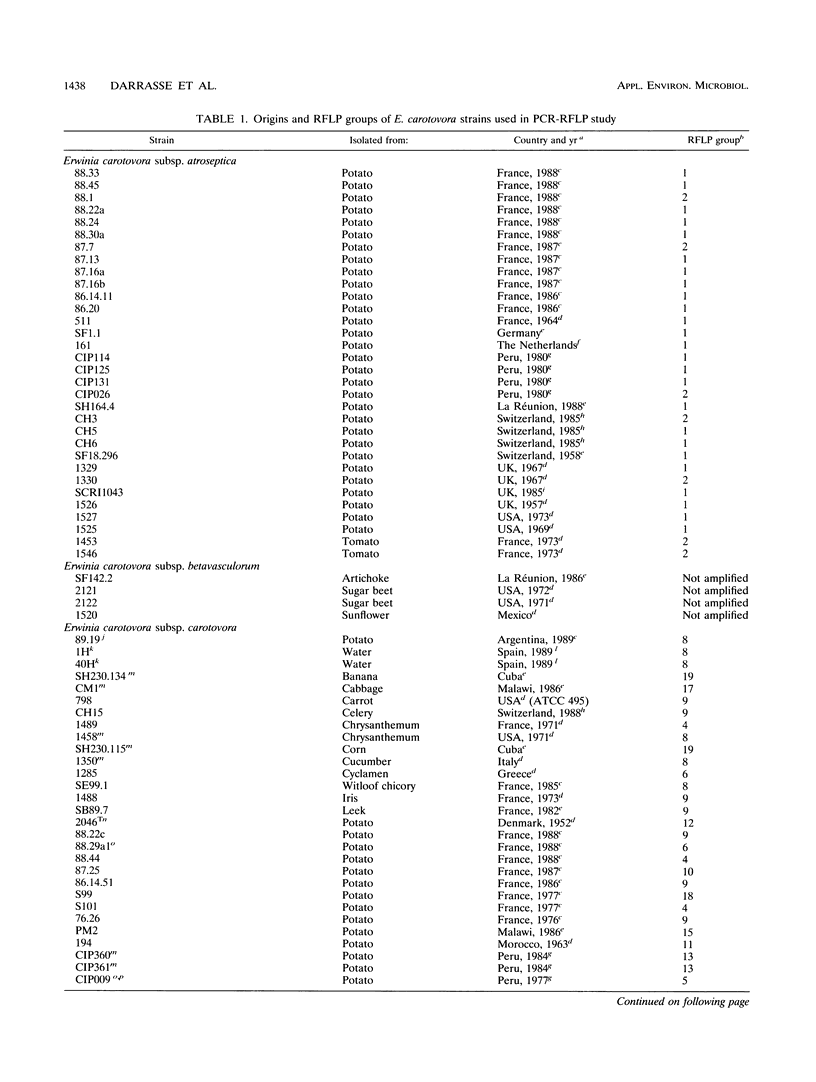
Using a sequenced pectate lyase-encoding gene (pel gene), we developed a PCR test for Erwinia carotovora. A set of primers allowed the amplification of a 434-bp fragment in E. carotovora strains. Among the 89 E. carotovora strains tested, only the Erwinia carotovora subsp. betavasculorum strains were not detected. A restriction fragment length polymorphism (RFLP) study was undertaken on the amplified fragment with seven endonucleases. The Sau3AI digestion pattern specifically identified the Erwinia carotovora subsp. atroseptica strains, and the whole set of data identified the Erwinia carotovora subsp. wasabiae strains. However, Erwinia carotovora subsp. carotovora and Erwinia carotovora subsp. odorifera could not be separated. Phenetic and phylogenic analyses of RFLP results showed E. carotovora subsp. atroseptica as a homogeneous group while E. carotovora subsp. carotovora and E. carotovora subsp. odorifera strains exhibited a genetic diversity that may result from a nonmonophyletic origin. The use of RFLP on amplified fragments in epidemiology and for diagnosis is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonierbale M. W., Plaisted R. L., Tanksley S. D. RFLP Maps Based on a Common Set of Clones Reveal Modes of Chromosomal Evolution in Potato and Tomato. Genetics. 1988 Dec;120(4):1095–1103. doi: 10.1093/genetics/120.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrasse A., Kotoujansky A., Bertheau Y. Isolation by genomic subtraction of DNA probes specific for Erwinia carotovora subsp. atroseptica. Appl Environ Microbiol. 1994 Jan;60(1):298–306. doi: 10.1128/aem.60.1.298-306.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton J. C., Sidebotham J. M., Gill D. R., Salmond G. P. Extracellular and periplasmic isoenzymes of pectate lyase from Erwinia carotovora subspecies carotovora belong to different gene families. Mol Microbiol. 1989 Dec;3(12):1785–1795. doi: 10.1111/j.1365-2958.1989.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Klotz L. C., Zimm B. H. Size of DNA determined by viscoelastic measurements: results on bacteriophages, Bacillus subtilis and Escherichia coli. J Mol Biol. 1972 Dec 30;72(3):779–800. doi: 10.1016/0022-2836(72)90191-x. [DOI] [PubMed] [Google Scholar]
- Kotoujansky A., Lemattre M., Boistard P. Utilization of a thermosensitive episome bearing transposon TN10 to isolate Hfr donor strains of Erwinia carotovora subsp. chrysanthemi. J Bacteriol. 1982 Apr;150(1):122–131. doi: 10.1128/jb.150.1.122-131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manulis S., Kobayashi D. Y., Keen N. T. Molecular cloning and sequencing of a pectate lyase gene from Yersinia pseudotuberculosis. J Bacteriol. 1988 Apr;170(4):1825–1830. doi: 10.1128/jb.170.4.1825-1830.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. P., Berman P. M., Allen C., Stromberg V. K., Lacy G. H., Mount M. S. Requirement for two or more Erwinia carotovora subsp. carotovora pectolytic gene products for maceration of potato tuber tissue by Escherichia coli. J Bacteriol. 1986 Jul;167(1):279–284. doi: 10.1128/jb.167.1.279-284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. Polymerase chain reaction: applications in environmental microbiology. Annu Rev Microbiol. 1991;45:137–161. doi: 10.1146/annurev.mi.45.100191.001033. [DOI] [PubMed] [Google Scholar]
- Trollinger D., Berry S., Belser W., Keen N. T. Cloning and characterization of a pectate lyase gene from Erwinia carotovora EC153. Mol Plant Microbe Interact. 1989 Jan-Feb;2(1):17–25. doi: 10.1094/mpmi-2-017. [DOI] [PubMed] [Google Scholar]