Abstract
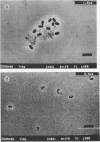
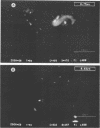
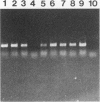
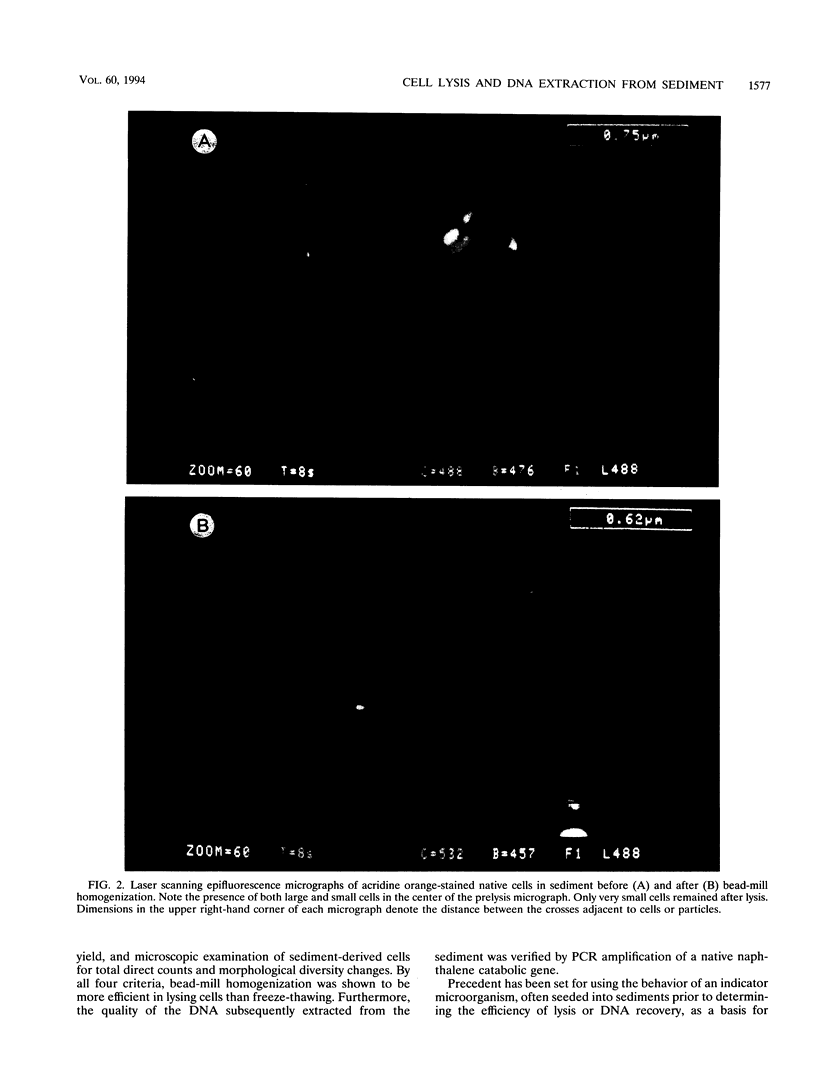
This study reports improvements in two of the key steps, lysis of indigenous cells and DNA purification, required for achieving a rapid nonselective protocol for extracting nucleic acids directly from sodium dodecyl sulfate (SDS)-treated sediment rich in organic matter. Incorporation of bead-mill homogenization into the DNA extraction procedure doubled the densitometrically determined DNA yield (11.8 micrograms of DNA.g [dry weight] of sediment-1) relative to incorporation of three cycles of freezing and thawing (5.2 micrograms of DNA.g [dry weight] of sediment-1). The improved DNA extraction efficiency was attributed to increased cell lysis, measured by viable counts of sediment microorganisms which showed that 2 and 8%, respectively, survived the bead-mill homogenization and freeze-thaw procedures. Corresponding measurements of suspensions of viable Bacillus endospores demonstrated that 2 and 94% of the initial number survived. Conventional, laser scanning epifluorescence phase-contrast, and differential interference-contrast microscopy revealed that small coccoid bacterial cells (1.2 to 0.3 micron long) were left intact after combined SDS and bead-mill homogenization of sediment samples. Estimates of the residual fraction of the fluorescently stained cell numbers indicated that 6% (2.2 x 10(8) cells.g [dry weight] of sediment-1) of the original population (3.8 x 10(9) cells.g [dry weight] of sediment-1) remained after treatment with SDS and bead-mill homogenization. Thus, lysis of total cells was less efficient than that of cells which could be cultured. The extracted DNA was used to successfully amplify nahR, the regulatory gene for naphthalene catabolism in Pseudomonas putida G7, by PCR.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbaszadegan M., Huber M. S., Gerba C. P., Pepper I. L. Detection of enteroviruses in groundwater with the polymerase chain reaction. Appl Environ Microbiol. 1993 May;59(5):1318–1324. doi: 10.1128/aem.59.5.1318-1324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R., Springer N., Ludwig W., Görtz H. D., Schleifer K. H. Identification in situ and phylogeny of uncultured bacterial endosymbionts. Nature. 1991 May 9;351(6322):161–164. doi: 10.1038/351161a0. [DOI] [PubMed] [Google Scholar]
- Balkwill D. L., Ghiorse W. C. Characterization of subsurface bacteria associated with two shallow aquifers in oklahoma. Appl Environ Microbiol. 1985 Sep;50(3):580–588. doi: 10.1128/aem.50.3.580-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin R. B., Velinsky D. J., Devereux R., Price W. A., Cifuentes L. A. Stable carbon isotope analysis of nucleic acids to trace sources of dissolved substrates used by estuarine bacteria. Appl Environ Microbiol. 1990 Jul;56(7):2012–2020. doi: 10.1128/aem.56.7.2012-2020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb R. W., Wagner-Döbler I. Detection of polychlorinated biphenyl degradation genes in polluted sediments by direct DNA extraction and polymerase chain reaction. Appl Environ Microbiol. 1993 Dec;59(12):4065–4073. doi: 10.1128/aem.59.12.4065-4073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., McCallum K., Davis A. A. Novel major archaebacterial group from marine plankton. Nature. 1992 Mar 12;356(6365):148–149. doi: 10.1038/356148a0. [DOI] [PubMed] [Google Scholar]
- Hahn D., Kester R., Starrenburg M. J., Akkermans A. D. Extraction of ribosomal RNA from soil for detection of Frankia with oligonucleotide probes. Arch Microbiol. 1990;154(4):329–335. doi: 10.1007/BF00276527. [DOI] [PubMed] [Google Scholar]
- Herrick J. B., Madsen E. L., Batt C. A., Ghiorse W. C. Polymerase chain reaction amplification of naphthalene-catabolic and 16S rRNA gene sequences from indigenous sediment bacteria. Appl Environ Microbiol. 1993 Mar;59(3):687–694. doi: 10.1128/aem.59.3.687-694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben W. E., Schroeter B. M., Calabrese V. G., Olsen R. H., Kukor J. K., Biederbeck V. O., Smith A. E., Tiedje J. M. Gene probe analysis of soil microbial populations selected by amendment with 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1992 Dec;58(12):3941–3948. doi: 10.1128/aem.58.12.3941-3948.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G., Vardund T., Skjerve E., Hornes E., Michaelsen T. E. Detection of pathogenic Yersinia enterocolitica in foods and water by immunomagnetic separation, nested polymerase chain reactions, and colorimetric detection of amplified DNA. Appl Environ Microbiol. 1993 Sep;59(9):2938–2944. doi: 10.1128/aem.59.9.2938-2944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesack W., Stackebrandt E. Occurrence of novel groups of the domain Bacteria as revealed by analysis of genetic material isolated from an Australian terrestrial environment. J Bacteriol. 1992 Aug;174(15):5072–5078. doi: 10.1128/jb.174.15.5072-5078.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen E. L., Sinclair J. L., Ghiorse W. C. In situ biodegradation: microbiological patterns in a contaminated aquifer. Science. 1991 May 10;252(5007):830–833. doi: 10.1126/science.2028258. [DOI] [PubMed] [Google Scholar]
- Moran M. A., Torsvik V. L., Torsvik T., Hodson R. E. Direct extraction and purification of rRNA for ecological studies. Appl Environ Microbiol. 1993 Mar;59(3):915–918. doi: 10.1128/aem.59.3.915-918.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C., Ponsonnet C., Paget E., Nesme X., Simonet P. Detection and enumeration of bacteria in soil by direct DNA extraction and polymerase chain reaction. Appl Environ Microbiol. 1992 Sep;58(9):2717–2722. doi: 10.1128/aem.58.9.2717-2722.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayler G. S., Layton A. C. Environmental application of nucleic acid hybridization. Annu Rev Microbiol. 1990;44:625–648. doi: 10.1146/annurev.mi.44.100190.003205. [DOI] [PubMed] [Google Scholar]
- Schell M. A., Sukordhaman M. Evidence that the transcription activator encoded by the Pseudomonas putida nahR gene is evolutionarily related to the transcription activators encoded by the Rhizobium nodD genes. J Bacteriol. 1989 Apr;171(4):1952–1959. doi: 10.1128/jb.171.4.1952-1959.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selenska S., Klingmüller W. DNA recovery and direct detection of Tn5 sequences from soil. Lett Appl Microbiol. 1991 Jul;13(1):21–24. doi: 10.1111/j.1472-765x.1991.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Steffan R. J., Goksøyr J., Bej A. K., Atlas R. M. Recovery of DNA from soils and sediments. Appl Environ Microbiol. 1988 Dec;54(12):2908–2915. doi: 10.1128/aem.54.12.2908-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbe C. C., Vahjen W. Interference of humic acids and DNA extracted directly from soil in detection and transformation of recombinant DNA from bacteria and a yeast. Appl Environ Microbiol. 1993 Aug;59(8):2657–2665. doi: 10.1128/aem.59.8.2657-2665.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsvik V., Goksøyr J., Daae F. L. High diversity in DNA of soil bacteria. Appl Environ Microbiol. 1990 Mar;56(3):782–787. doi: 10.1128/aem.56.3.782-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Detection of low numbers of bacterial cells in soils and sediments by polymerase chain reaction. Appl Environ Microbiol. 1992 Feb;58(2):754–757. doi: 10.1128/aem.58.2.754-757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Rapid method for direct extraction of DNA from soil and sediments. Appl Environ Microbiol. 1991 Apr;57(4):1070–1074. doi: 10.1128/aem.57.4.1070-1074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Rapid method for separation of bacterial DNA from humic substances in sediments for polymerase chain reaction. Appl Environ Microbiol. 1992 Jul;58(7):2292–2295. doi: 10.1128/aem.58.7.2292-2295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Park M. J., Olson B. H. Rapid method for direct extraction of mRNA from seeded soils. Appl Environ Microbiol. 1991 Mar;57(3):765–768. doi: 10.1128/aem.57.3.765-768.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. M., Weller R., Bateson M. M. 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature. 1990 May 3;345(6270):63–65. doi: 10.1038/345063a0. [DOI] [PubMed] [Google Scholar]
- Winans S. C., Rooks M. J. Sensitive, economical laboratory photodocumentation using a standard video camera and thermal printer. Biotechniques. 1993 Jun;14(6):902–905. [PubMed] [Google Scholar]
- You I. S., Ghosal D., Gunsalus I. C. Nucleotide sequence of plasmid NAH7 gene nahR and DNA binding of the nahR product. J Bacteriol. 1988 Dec;170(12):5409–5415. doi: 10.1128/jb.170.12.5409-5415.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]