Abstract
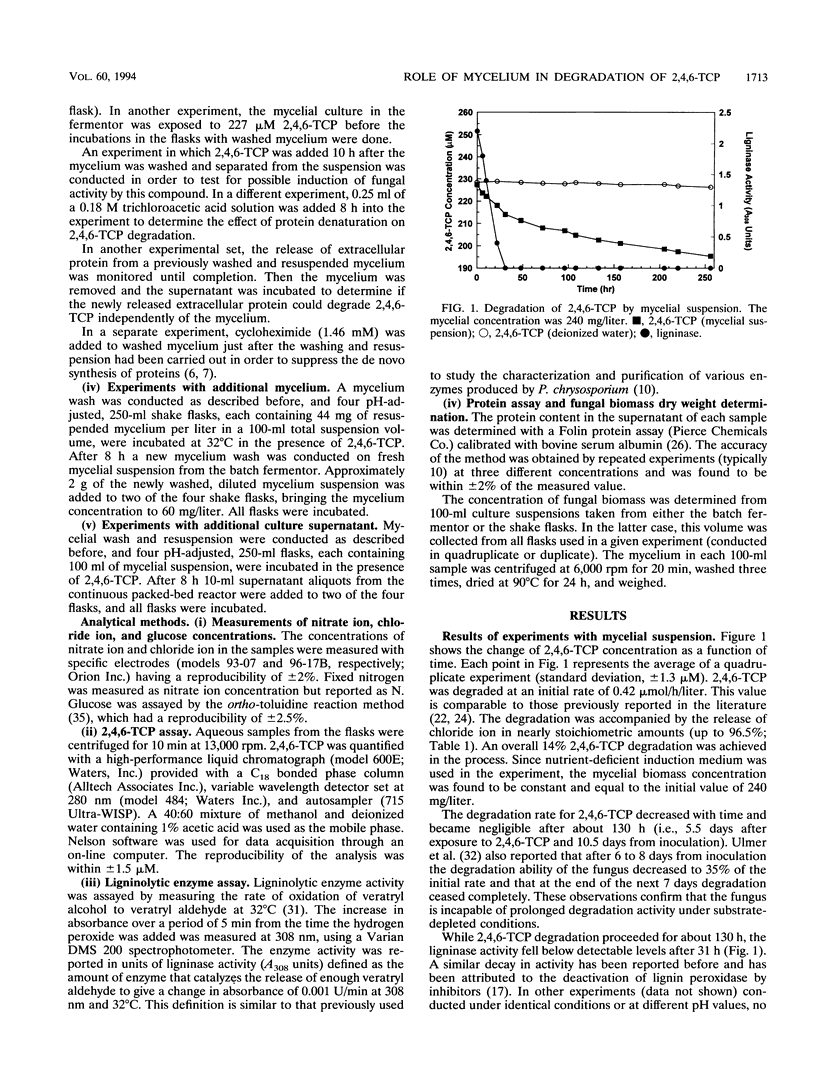
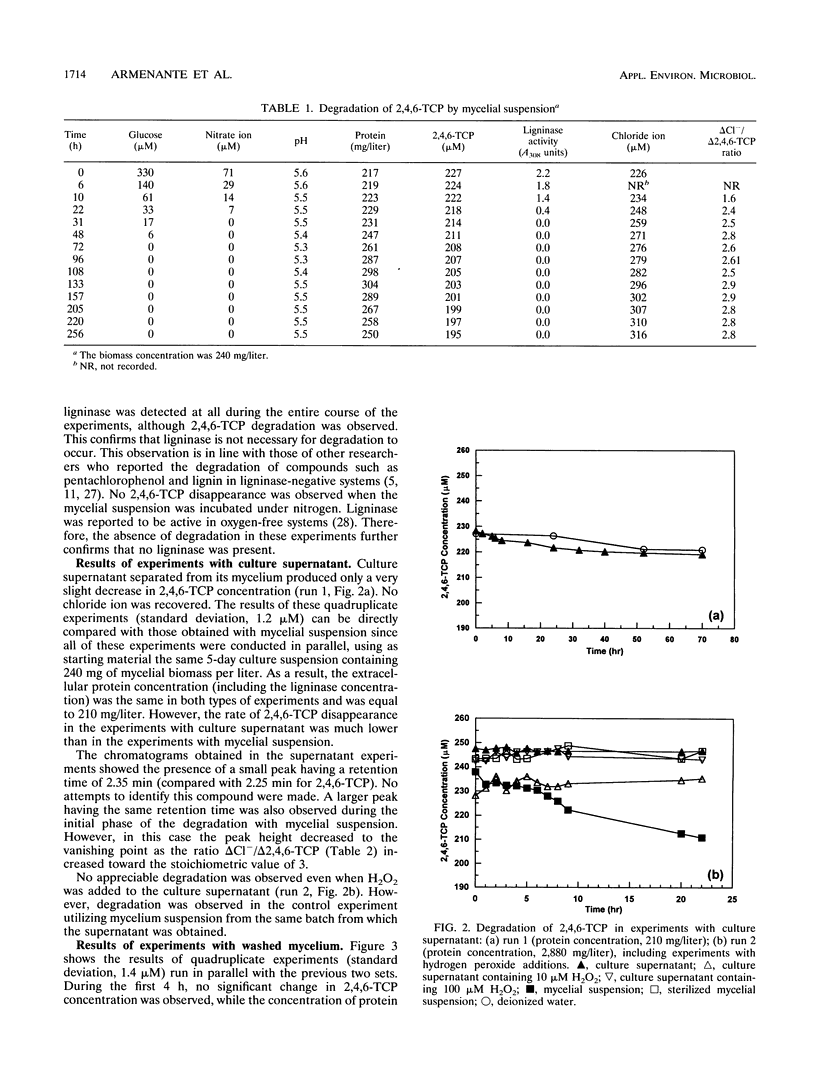
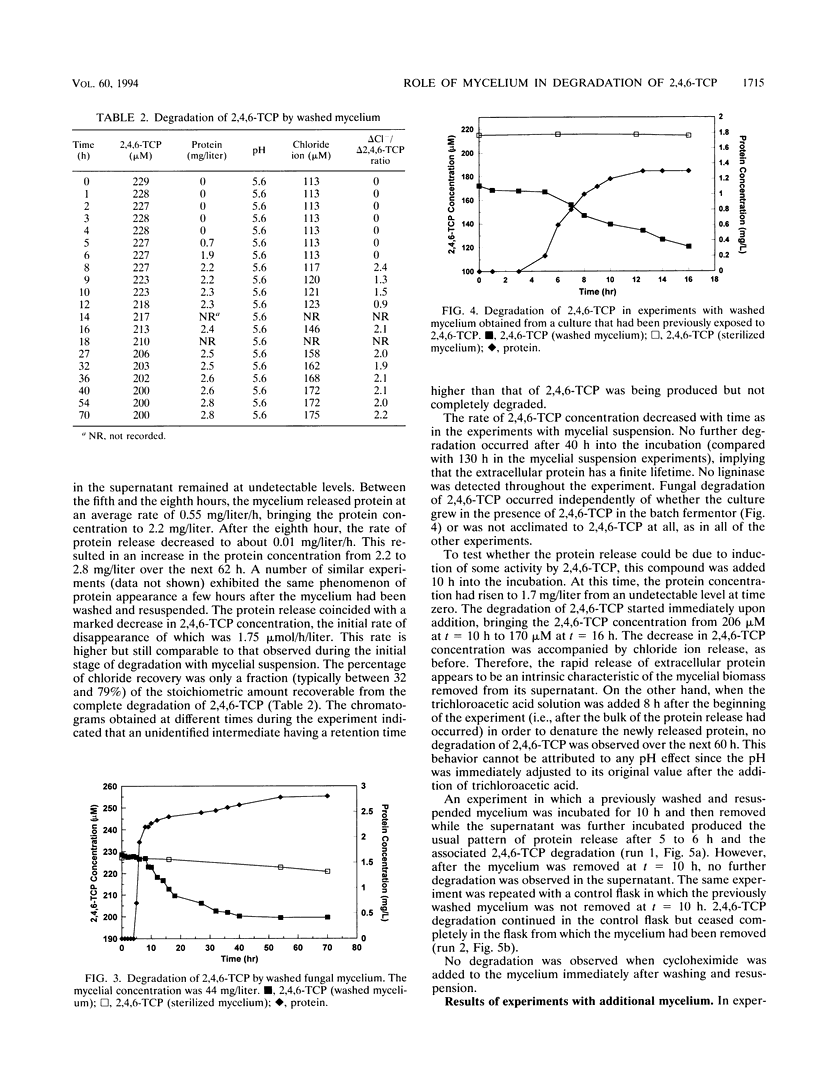
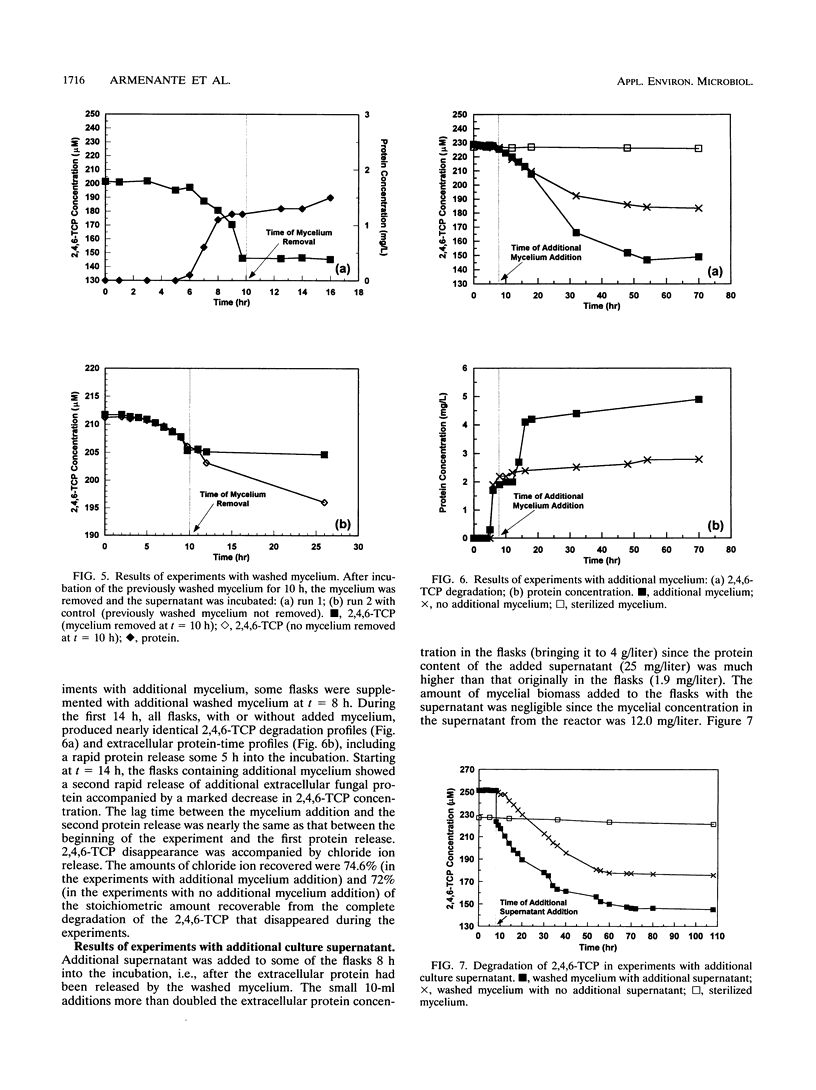
The biodegradation of 2,4,6-trichlorophenol (2,4,6-TCP) by Phanerochaete chrysosporium was studied in batch systems. In experiments with mycelial suspension, the degradation of 2,4,6-TCP was found to occur in the absence of ligninase. Chloride ion was recovered in nearly stoichiometric amounts at the end of the process. The microorganism did not retain its degradation ability for more than 6 days under substrate-deficient conditions. Neither the mycelium nor the extracellular protein alone could degrade 2,4,6-TCP; both were required for complete degradation to occur. In experiments in which 2,4,6-TCP was exposed to the culture supernatant separated from its mycelium, negligible degradation was obtained and no chloride ion was recovered. No degradation was observed even when the supernatant was supplemented with hydrogen peroxide as a possible cosubstrate. In experiments performed with washed mycelium separated from its supernatant, no degradation took place until the mycelium released additional extracellular protein 5 to 6 h into the incubation. Additions of washed mycelium separated from its supernatant to active cultures also produced an increase in the rate of degradation in correspondence with the protein release. The protein release was independent of the presence of 2,4,6-TCP. The addition of cycloheximide to inhibit the synthesis of de novo proteins completely suppressed the release of protein by the mycelium and resulted in no 2,4,6-TCP degradation. Additions of culture supernatants containing a high concentration of extracellular protein to active cultures produced an increase in the rate of 2,4,6-TCP degradation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boominathan K., Dass S. B., Randall T. A., Kelley R. L., Reddy C. A. Lignin peroxidase-negative mutant of the white-rot basidiomycete Phanerochaete chrysosporium. J Bacteriol. 1990 Jan;172(1):260–265. doi: 10.1128/jb.172.1.260-265.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A. Biodegradation of polycyclic hydrocarbons by Phanerochaete chrysosporium. Appl Environ Microbiol. 1989 Jan;55(1):154–158. doi: 10.1128/aem.55.1.154-158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Tien M., Wright D., Aust S. D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985 Jun 21;228(4706):1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- Datta A. Purification and characterization of a novel protease from solid substrate cultures of Phanerochaete chrysosporium. J Biol Chem. 1992 Jan 15;267(2):728–732. [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Factors Involved in the Regulation of a Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Feb;49(2):299–304. doi: 10.1128/aem.49.2.299-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K., Farrell R. L. Role of Veratryl Alcohol in Regulating Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1986 Aug;52(2):251–254. doi: 10.1128/aem.52.2.251-254.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel K. E., Gai W. Z., Green B., Moen M. A. Oxidative degradation of phenanthrene by the ligninolytic fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Jun;58(6):1832–1838. doi: 10.1128/aem.58.6.1832-1838.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel K. E., Kalyanaraman B., Kirk T. K. Oxidation of polycyclic aromatic hydrocarbons and dibenzo[p]-dioxins by Phanerochaete chrysosporium ligninase. J Biol Chem. 1986 Dec 25;261(36):16948–16952. [PubMed] [Google Scholar]
- Joshi D. K., Gold M. H. Degradation of 2,4,5-trichlorophenol by the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1993 Jun;59(6):1779–1785. doi: 10.1128/aem.59.6.1779-1785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R. L., Reddy C. A. Purification and characterization of glucose oxidase from ligninolytic cultures of Phanerochaete chrysosporium. J Bacteriol. 1986 Apr;166(1):269–274. doi: 10.1128/jb.166.1.269-274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamar R. T., Dietrich D. M. In Situ Depletion of Pentachlorophenol from Contaminated Soil by Phanerochaete spp. Appl Environ Microbiol. 1990 Oct;56(10):3093–3100. doi: 10.1128/aem.56.10.3093-3100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileski G. J., Bumpus J. A., Jurek M. A., Aust S. D. Biodegradation of pentachlorophenol by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1988 Dec;54(12):2885–2889. doi: 10.1128/aem.54.12.2885-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J. B., Selby A. L., Freeman J. P., Evans F. E., Cerniglia C. E. Metabolism of phenanthrene by Phanerochaete chrysosporium. Appl Environ Microbiol. 1991 Nov;57(11):3310–3316. doi: 10.1128/aem.57.11.3310-3316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer D. C., Leisola M. S., Schmidt B. H., Fiechter A. Rapid Degradation of Isolated Lignins by Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Jun;45(6):1795–1801. doi: 10.1128/aem.45.6.1795-1801.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Brock B. J., Joshi D. K., Gold M. H. Degradation of 2,4-dinitrotoluene by the lignin-degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Jan;58(1):221–228. doi: 10.1128/aem.58.1.221-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Gold M. H. Degradation of 2,4-dichlorophenol by the lignin-degrading fungus Phanerochaete chrysosporium. J Bacteriol. 1991 Jan;173(1):345–352. doi: 10.1128/jb.173.1.345-352.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZENDER R. [An automatic micro-method for the quantitative analysis of aldohexoses in biological fluids by o-toluidine]. Clin Chim Acta. 1963 May;8:351–358. doi: 10.1016/0009-8981(63)90071-8. [DOI] [PubMed] [Google Scholar]


