Abstract
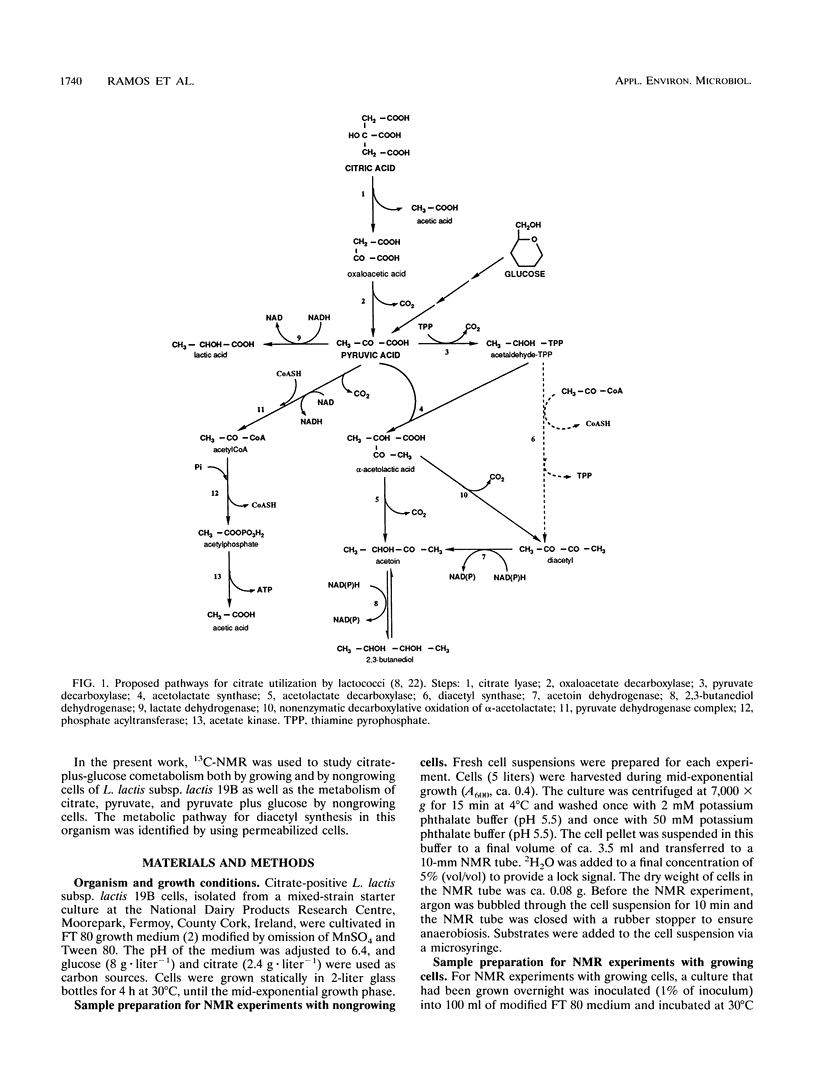
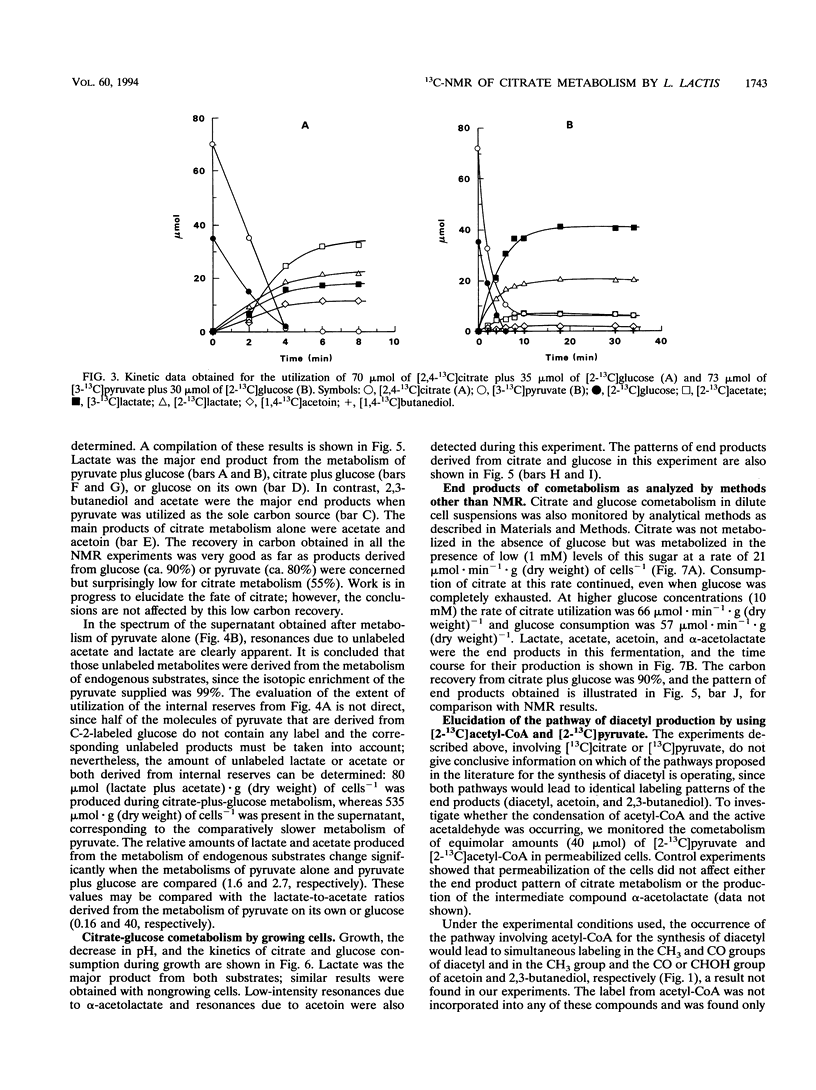
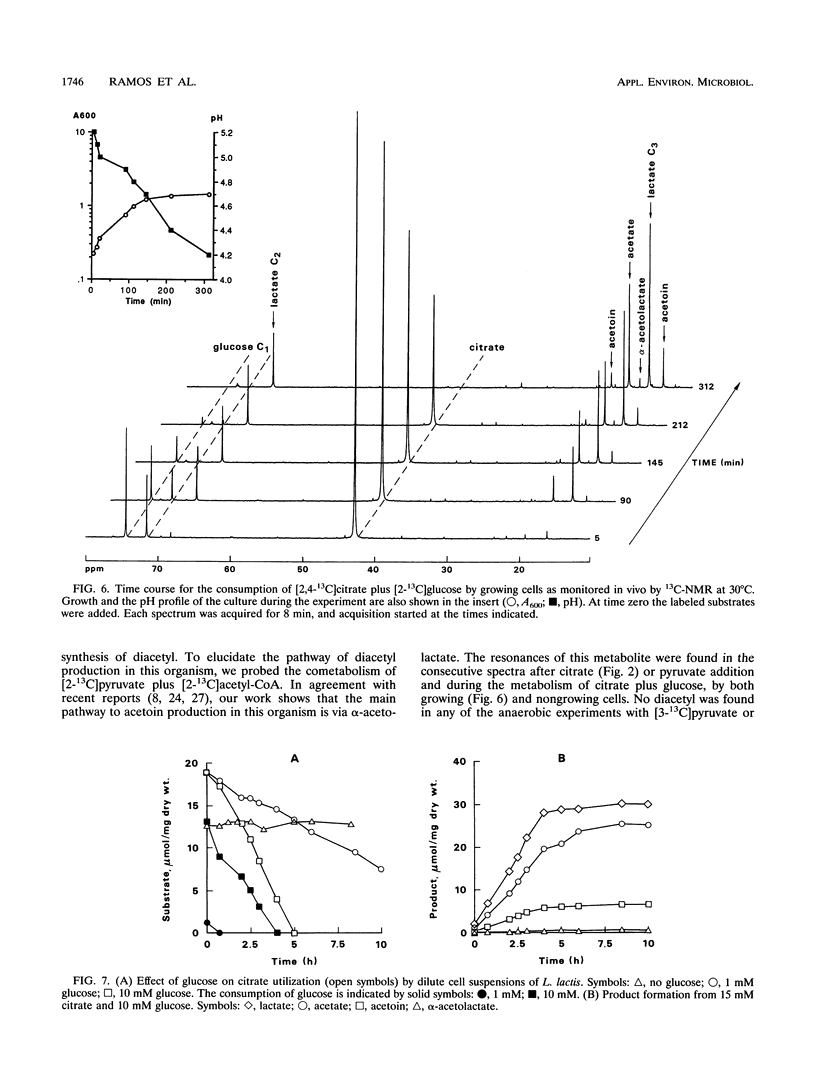
13C nuclear magnetic resonance (13C-NMR) was used to investigate the metabolism of citrate plus glucose and pyruvate plus glucose by nongrowing cells of Lactococcus lactis subsp. lactis 19B under anaerobic conditions. The metabolism of citrate plus glucose during growth was also monitored directly by in vivo NMR. Although pyruvate is a common intermediate metabolite in the metabolic pathways of both citrate and glucose, the origin of the carbon atoms in the fermentation products was determined by using selectively labeled substrates, e.g., [2,4-13C]citrate, [3-13C]pyruvate, and [2-13C]glucose. The presence of an additional substrate caused a considerable stimulation in the rates of substrate utilization, and the pattern of end products was changed. Acetate plus acetoin and butanediol represented more than 80% (molar basis) of the end products of the metabolism of citrate (or pyruvate) alone, but when glucose was also added, 80% of the citrate (or pyruvate) was converted to lactate. This result can be explained by the activation of lactate dehydrogenase by fructose 1,6-bisphosphate, an intermediate in glucose metabolism. The effect of different concentrations of glucose on the metabolism of citrate by dilute cell suspensions was also probed by using analytical methods other than NMR. Pyruvate dehydrogenase (but not pyruvate formate-lyase) was active in the conversion of pyruvate to acetyl coenzyme A. α-Acetolactate was detected as an intermediate metabolite of citrate or pyruvate metabolism, and the labeling pattern of the end products agrees with the α-acetolactate pathway. It was demonstrated that the contribution of the acetyl coenzyme A pathway for the synthesis of diacetyl, should it exist, is lower than 10%. Evidence for the presence of internal carbon reserves in L. lactis is presented.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbe K., Takahashi S., Yamada T. Involvement of oxygen-sensitive pyruvate formate-lyase in mixed-acid fermentation by Streptococcus mutans under strictly anaerobic conditions. J Bacteriol. 1982 Oct;152(1):175–182. doi: 10.1128/jb.152.1.175-182.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavin J. F., Prevost H., Lin J., Schmitt P., Divies C. Medium for Screening Leuconostoc oenos Strains Defective in Malolactic Fermentation. Appl Environ Microbiol. 1989 Mar;55(3):751–753. doi: 10.1128/aem.55.3.751-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan T. M., O'dowd M., Mellerick D. Effects of pH and Sugar on Acetoin Production from Citrate by Leuconostoc lactis. Appl Environ Microbiol. 1981 Jan;41(1):1–8. doi: 10.1128/aem.41.1.1-8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L. Properties of 2,3-Butanediol Dehydrogenases from Lactococcus lactis subsp. lactis in Relation to Citrate Fermentation. Appl Environ Microbiol. 1990 Jun;56(6):1656–1665. doi: 10.1128/aem.56.6.1656-1665.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAY H. S. Planning and equipping a disease diagnostic control laboratory. Lab Anim Care. 1963 Jun;2:431–441. [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Takahashi M., Suzuki H. Acetoin Fermentation by Citrate-Positive Lactococcus lactis subsp. lactis 3022 Grown Aerobically in the Presence of Hemin or Cu. Appl Environ Microbiol. 1990 Sep;56(9):2644–2649. doi: 10.1128/aem.56.9.2644-2649.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoep J. L., Teixeira de Mattos M. J., Starrenburg M. J., Hugenholtz J. Isolation, characterization, and physiological role of the pyruvate dehydrogenase complex and alpha-acetolactate synthase of Lactococcus lactis subsp. lactis bv. diacetylactis. J Bacteriol. 1992 Jul;174(14):4838–4841. doi: 10.1128/jb.174.14.4838-4841.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoep J. L., de Graef M. R., Teixeira de Mattos M. J., Neijssel O. M. Pyruvate catabolism during transient state conditions in chemostat cultures of Enterococcus faecalis NCTC 775: importance of internal pyruvate concentrations and NADH/NAD+ ratios. J Gen Microbiol. 1992 Oct;138(10):2015–2020. doi: 10.1099/00221287-138-10-2015. [DOI] [PubMed] [Google Scholar]
- Speckman R. A., Collins E. B. Diacetyl biosynthesis in Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol. 1968 Jan;95(1):174–180. doi: 10.1128/jb.95.1.174-180.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckman R. A., Collins E. B. Incorporation of radioactive acetate into diacetyl by Streptococcus diacetilactis. Appl Microbiol. 1973 Nov;26(5):744–746. doi: 10.1128/am.26.5.744-746.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrenburg M. J., Hugenholtz J. Citrate Fermentation by Lactococcus and Leuconostoc spp. Appl Environ Microbiol. 1991 Dec;57(12):3535–3540. doi: 10.1128/aem.57.12.3535-3540.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Degradation of cell constituents by starved Streptococcus lactis in relation to survival. J Gen Microbiol. 1969 Nov;58(3):347–362. doi: 10.1099/00221287-58-3-347. [DOI] [PubMed] [Google Scholar]
- Thornhill P. J., Cogan T. M. Use of gas-liquid chromatography to determine the end products of growth of lactic Acid bacteria. Appl Environ Microbiol. 1984 Jun;47(6):1250–1254. doi: 10.1128/aem.47.6.1250-1254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga da Cunha M., Foster M. A. Sugar-glycerol cofermentations in lactobacilli: the fate of lactate. J Bacteriol. 1992 Feb;174(3):1013–1019. doi: 10.1128/jb.174.3.1013-1019.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Da-Cunha M., Firme P., Romão M. V., Santos H. Application of C Nuclear Magnetic Resonance To Elucidate the Unexpected Biosynthesis of Erythritol by Leuconostoc oenos. Appl Environ Microbiol. 1992 Jul;58(7):2271–2279. doi: 10.1128/aem.58.7.2271-2279.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhue W. M., Tjan F. S. Study of the Citrate Metabolism of Lactococcus lactis subsp. lactis Biovar Diacetylactis by Means of C Nuclear Magnetic Resonance. Appl Environ Microbiol. 1991 Nov;57(11):3371–3377. doi: 10.1128/aem.57.11.3371-3377.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


