Abstract
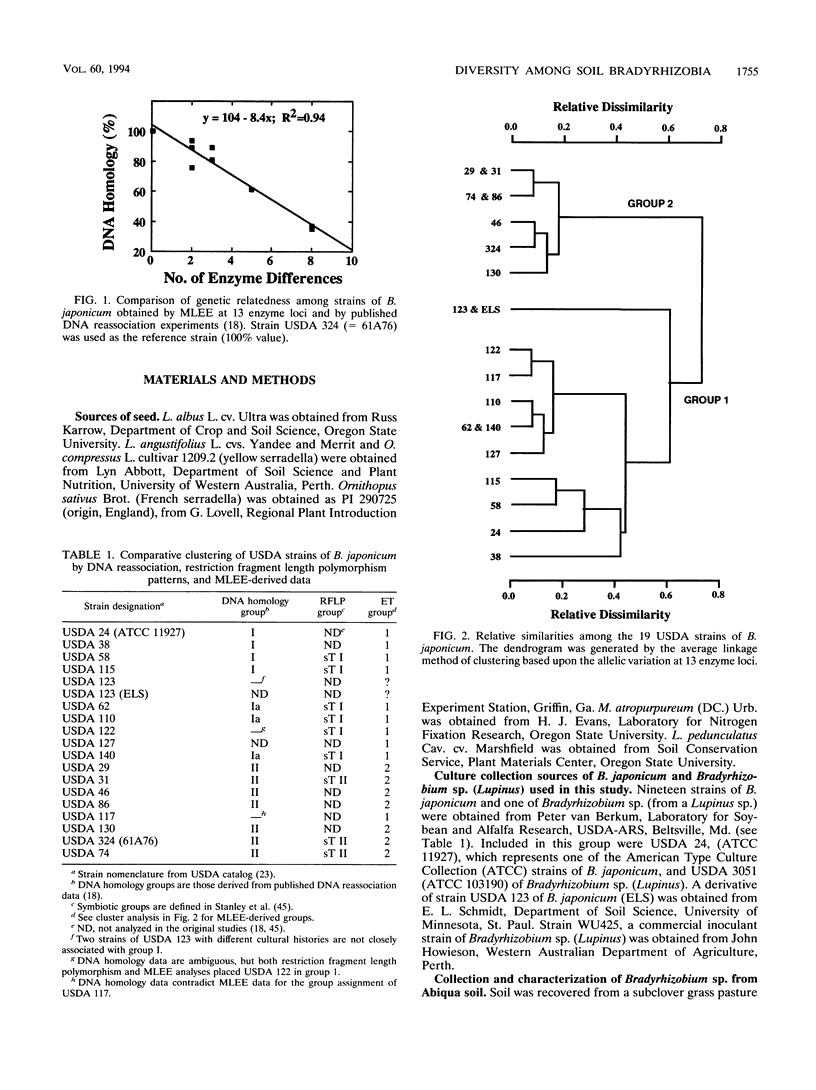
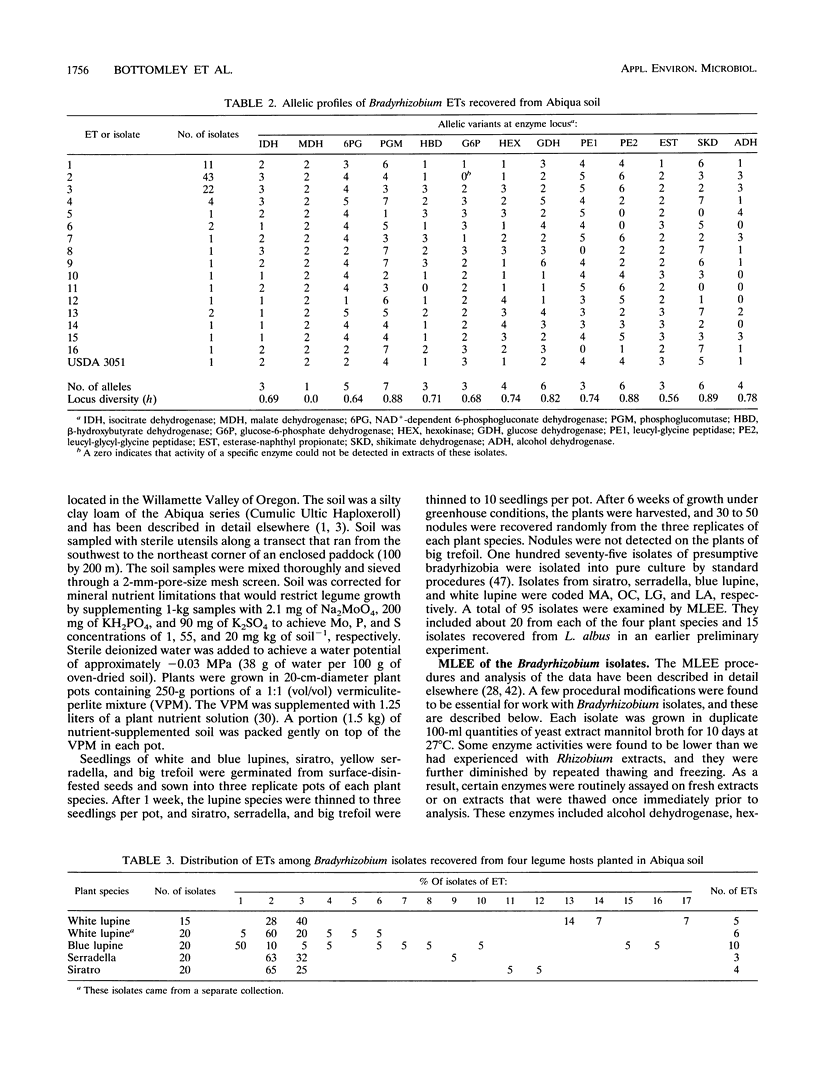
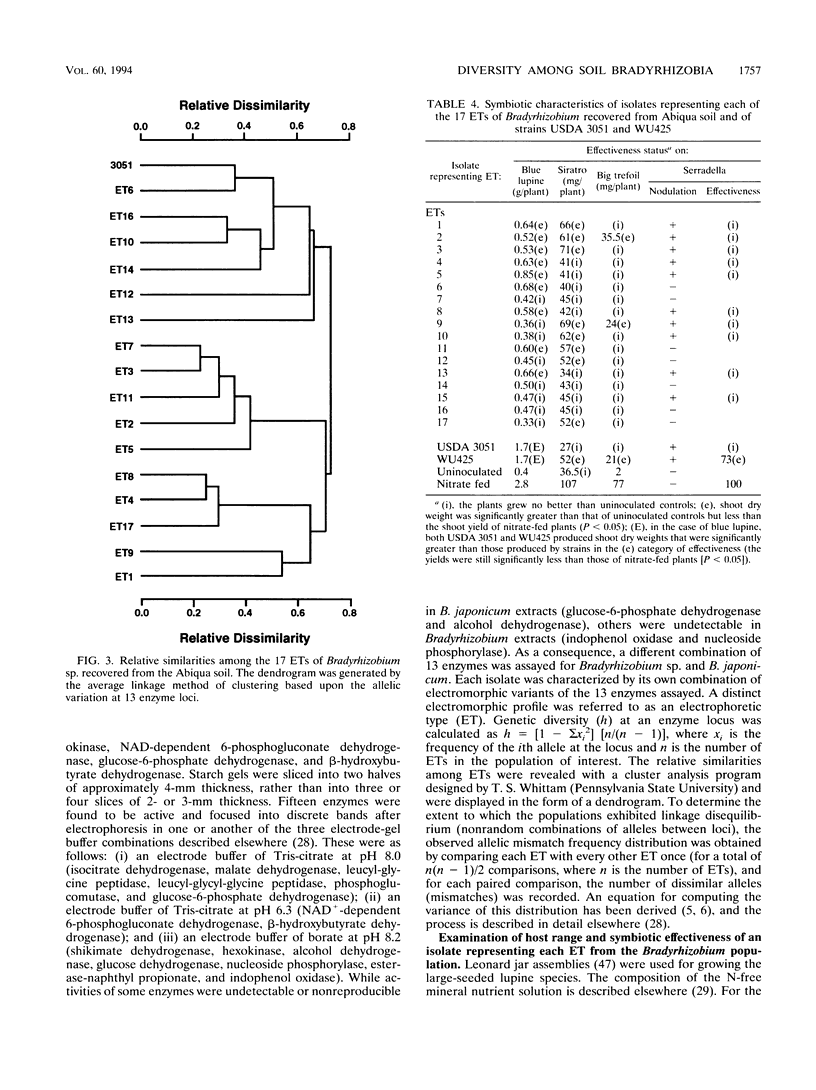
We examined the genetic structure and symbiotic characteristics of Bradyrhizobium isolates recovered from four legume species (Lupinus albus [white lupine], Lupinus angustifolius [blue lupine], Ornithopus compressus [yellow serradella], and Macroptilium atropurpureum [sirato]) grown in an Oregon soil. We established that multilocus enzyme electrophoresis (MLEE) can provide insights into the genetic relatedness among Bradyrhizobium strains by showing a positive correlation (r2 = ≥0.90) between the relatedness of Bradyrhizobium japonicum strains determined by MLEE at 13 enzyme loci and that determined by other workers using either DNA-DNA hybridization or DNA sequence divergence estimates. MLEE identified 17 electrophoretic types (ETs) among 95 Bradyrhizobium isolates recovered from the four hosts. Although the overall genetic diversity among the ETs (H = 0.69) is one of the largest measured to date in a local population of any soilborne bacterial species, there was no evidence of multilocus structure (linkage disequilibrium) within the population. The majority of the isolates (73%) were represented by two closely related ETs (2 and 3) which dominated the root nodules of white lupine, serradella, and siratro. In contrast, ET1 dominated nodules of blue lupine. Although representative isolates from all of the 17 ETs nodulated siratro, white lupine, blue lupine, and big trefoil (Lotus pedunculatus), they were either completely ineffective or poorly effective at fixing nitrogen on these hosts. Despite the widespread use of serradella as a surrogate host for lupine-nodulating bradyrhizobia, 7 of the 17 ETs did not nodulate this host, and the remaining 10 ETs were ineffective at fixing nitrogen.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendras A. S., Bottomley P. J. Influence of Lime and Phosphate on Nodulation of Soil-Grown Trifolium subterraneum L. by Indigenous Rhizobium trifolii. Appl Environ Microbiol. 1987 Sep;53(9):2090–2097. doi: 10.1128/aem.53.9.2090-2097.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley P. J., Dughri M. H. Population Size and Distribution of Rhizobium leguminosarum bv. trifolii in Relation to Total Soil Bacteria and Soil Depth. Appl Environ Microbiol. 1989 Apr;55(4):959–964. doi: 10.1128/aem.55.4.959-964.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. H., Feldman M. W., Nevo E. Multilocus Structure of Natural Populations of HORDEUM SPONTANEUM. Genetics. 1980 Oct;96(2):523–536. doi: 10.1093/genetics/96.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. H., Feldman M. W. Population structure of multilocus associations. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5913–5916. doi: 10.1073/pnas.78.9.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demezas D. H., Reardon T. B., Watson J. M., Gibson A. H. Genetic Diversity among Rhizobium leguminosarum bv. Trifolii Strains Revealed by Allozyme and Restriction Fragment Length Polymorphism Analyses. Appl Environ Microbiol. 1991 Dec;57(12):3489–3495. doi: 10.1128/aem.57.12.3489-3495.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eardly B. D., Materon L. A., Smith N. H., Johnson D. A., Rumbaugh M. D., Selander R. K. Genetic structure of natural populations of the nitrogen-fixing bacterium Rhizobium meliloti. Appl Environ Microbiol. 1990 Jan;56(1):187–194. doi: 10.1128/aem.56.1.187-194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt M. M., Baldwin I. L., Fred E. B. Studies of the Root-Nodule Organism of Lupinus. J Bacteriol. 1931 Apr;21(4):273–285. doi: 10.1128/jb.21.4.273-285.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M., Hennecke H. Conservation of a symbiotic DNA region in soybean root nodule bacteria. Appl Environ Microbiol. 1987 Sep;53(9):2253–2255. doi: 10.1128/aem.53.9.2253-2255.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber T. A., Agarwal A. K., Keister D. L. Extracellular polysaccharide composition, ex planta nitrogenase activity, and DNA homology in Rhizobium japonicum. J Bacteriol. 1984 Jun;158(3):1168–1171. doi: 10.1128/jb.158.3.1168-1171.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istock C. A., Duncan K. E., Ferguson N., Zhou X. Sexuality in a natural population of bacteria--Bacillus subtilis challenges the clonal paradigm. Mol Ecol. 1992 Aug;1(2):95–103. doi: 10.1111/j.1365-294x.1992.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Jenkins M. B., Virginia R. A., Jarrell W. M. Rhizobial Ecology of the Woody Legume Mesquite (Prosopis glandulosa) in the Sonoran Desert. Appl Environ Microbiol. 1987 Jan;53(1):36–40. doi: 10.1128/aem.53.1.36-40.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie A. C. Relationships among rhizobia from native Australian legumes. Appl Environ Microbiol. 1983 Jun;45(6):1822–1828. doi: 10.1128/aem.45.6.1822-1828.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K., Strain S. R., de Bruijn F. J., Bottomley P. J. Genotypic and Phenotypic Comparisons of Chromosomal Types within an Indigenous Soil Population of Rhizobium leguminosarum bv. trifolii. Appl Environ Microbiol. 1994 Feb;60(2):416–426. doi: 10.1128/aem.60.2.416-426.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K., Wanjage F. N., Bottomley P. J. Symbiotic Characteristics of Rhizobium leguminosarum bv. trifolii Isolates Which Represent Major and Minor Nodule-Occupying Chromosomal Types of Field-Grown Subclover (Trifolium subterraneum L.). Appl Environ Microbiol. 1994 Feb;60(2):427–433. doi: 10.1128/aem.60.2.427-433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K., Yap K., Dashti N., Bottomley P. J. Serological and Ecological Characteristics of a Nodule-Dominant Serotype from an Indigenous Soil Population of Rhizobium leguminosarum bv. trifolii. Appl Environ Microbiol. 1994 Feb;60(2):408–415. doi: 10.1128/aem.60.2.408-415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Romero E., Segovia L., Mercante F. M., Franco A. A., Graham P., Pardo M. A. Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int J Syst Bacteriol. 1991 Jul;41(3):417–426. doi: 10.1099/00207713-41-3-417. [DOI] [PubMed] [Google Scholar]
- O'Rourke M., Stevens E. Genetic structure of Neisseria gonorrhoeae populations: a non-clonal pathogen. J Gen Microbiol. 1993 Nov;139(11):2603–2611. doi: 10.1099/00221287-139-11-2603. [DOI] [PubMed] [Google Scholar]
- Pinero D., Martinez E., Selander R. K. Genetic diversity and relationships among isolates of Rhizobium leguminosarum biovar phaseoli. Appl Environ Microbiol. 1988 Nov;54(11):2825–2832. doi: 10.1128/aem.54.11.2825-2832.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia L., Piñero D., Palacios R., Martínez-Romero E. Genetic structure of a soil population of nonsymbiotic Rhizobium leguminosarum. Appl Environ Microbiol. 1991 Feb;57(2):426–433. doi: 10.1128/aem.57.2.426-433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia L., Young J. P., Martínez-Romero E. Reclassification of American Rhizobium leguminosarum biovar phaseoli type I strains as Rhizobium etli sp. nov. Int J Syst Bacteriol. 1993 Apr;43(2):374–377. doi: 10.1099/00207713-43-2-374. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. M., Smith N. H., O'Rourke M., Spratt B. G. How clonal are bacteria? Proc Natl Acad Sci U S A. 1993 May 15;90(10):4384–4388. doi: 10.1073/pnas.90.10.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza V., Nguyen T. T., Hudson R. R., Piñero D., Lenski R. E. Hierarchical analysis of linkage disequilibrium in Rhizobium populations: evidence for sex? Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8389–8393. doi: 10.1073/pnas.89.17.8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Brown G. G., Verma D. P. Slow-growing Rhizobium japonicum comprises two highly divergent symbiotic types. J Bacteriol. 1985 Jul;163(1):148–154. doi: 10.1128/jb.163.1.148-154.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. P., Demetriou L., Apte R. G. Rhizobium Population Genetics: Enzyme Polymorphism in Rhizobium leguminosarum from Plants and Soil in a Pea Crop. Appl Environ Microbiol. 1987 Feb;53(2):397–402. doi: 10.1128/aem.53.2.397-402.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


