Abstract
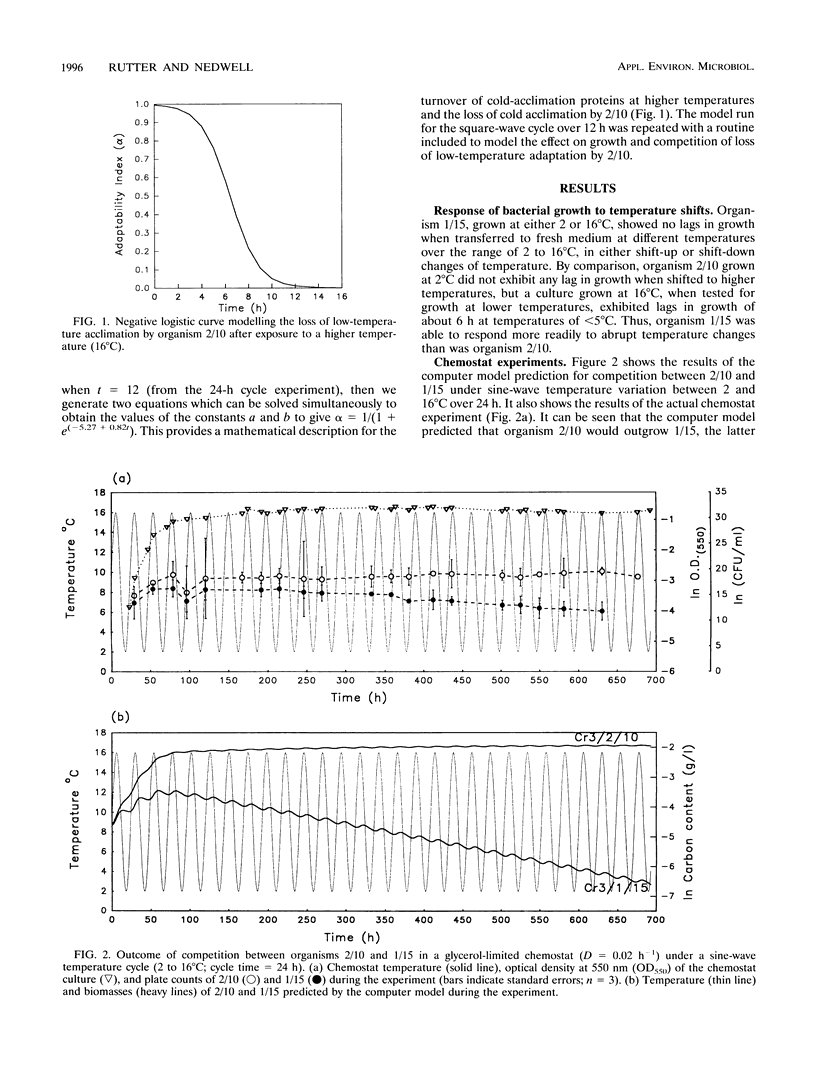
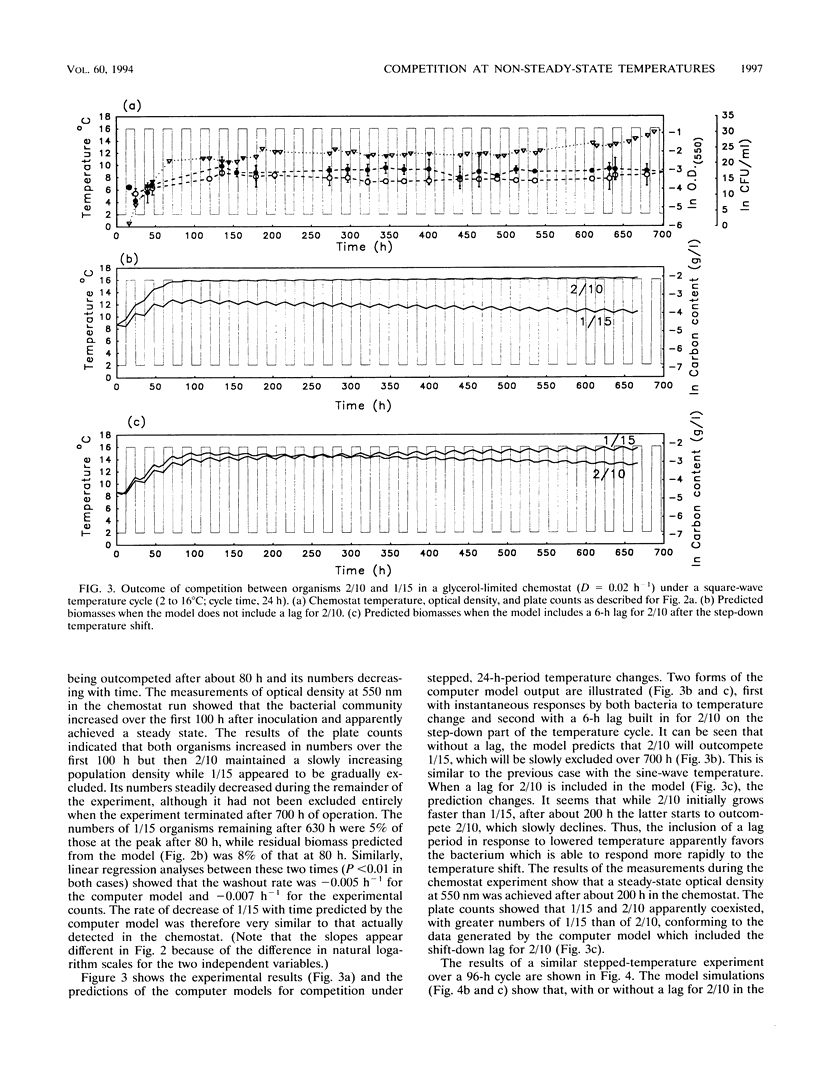
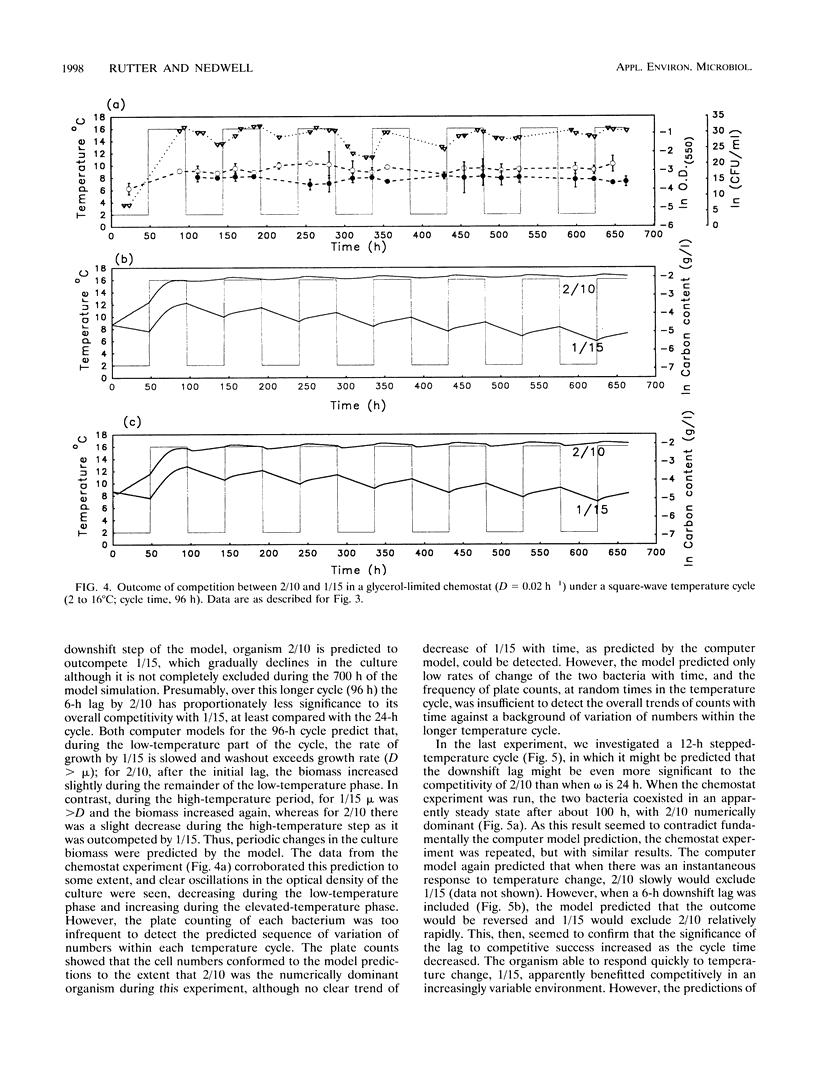
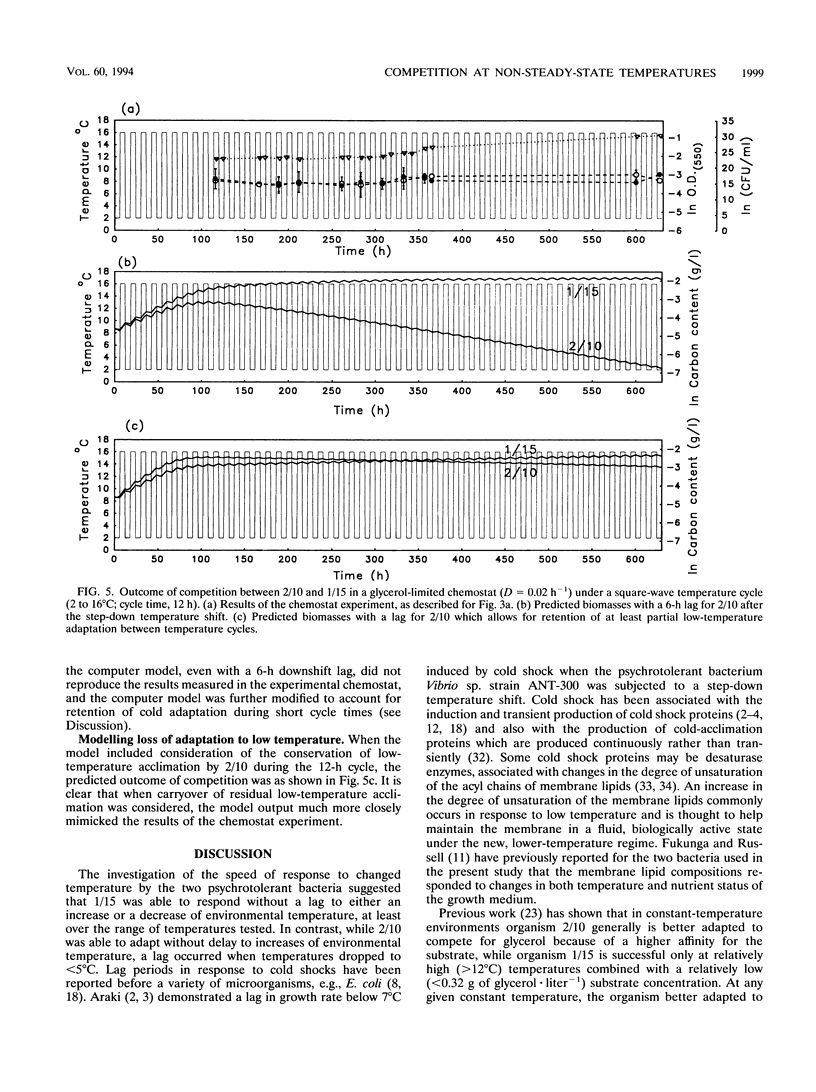
Competition between two psychrotolerant bacteria was examined in glycerol-limited chemostat experiments subjected to non-steady-state conditions of temperature. One bacterium, a Brevibacterium sp. strain designated CR3/1/15, responded rapidly to temperature change, while a second, Hydrogenophaga pseudoflava, designated CR3/2/10, exhibited a lag in growth after a shift-down during a square-wave temperature cycle but not after a shift-up. The effects on competition and survival by these bacteria of both sine-wave and square-wave temperature changes between 2 and 16 degrees C over a 24-h cycle time were examined, as well as square-wave cycles over 12 and 96 h. The changing proportion of each bacterium in the chemostat was determined by plate counting at regular intervals. Under a sine-wave temperature cycle H. psedoflava outcompeted the Brevibacterium sp., but under square-wave temperature cycles the two bacteria coexisted because the lag by H. pseudoflava after the temperature shift-down favored the faster-responding Brevibacterium sp. The two bacteria thus exhibited different survival strategies, with H. pseudoflava adapted to effective competition under steady-state conditions and the Brevibacterium sp. adapted to rapid adaptation and survival in a changing environment. The degree of perturbation of the bacteria, expressed as a temperature challenge index (delta temp/delta time), was greater under a square-wave temperature cycle than under a sine-wave cycle of equivalent amplitude and frequency, and higher-temperature challenge favored the Brevibacterium sp. A computer model was developed to examine competition between the bacteria in transient environments. The frequency of the temperature cycle influenced competition, as with a longer cycle (96 h) the significance of the lag by H. pseudoflava decreased compared with that of a 24-h cycle, and H. pseudoflava predominated in a mixed culture with a 96-h cycle. The shift-down lag by H. pseudoflava, during which it adapted to low temperature, disadvantaged it in a changing temperature environment, but at a short cycle time (12 h) this disadvantage was countered by the incomplete loss of low-temperature adaptation between cycles and thus the carryover of some low-temperature adaptation. Also, it was demonstrated that, as well as consideration of the effect of temperature changes on inducing lags in growth, the loss of adaptation to low temperature between cycles had to be taken into account in the computer model if it was to reproduce the trends in the experimental data.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki T. An analysis of the effect of changes in growth temperature on proteolysis in vivo in the psychrophilic bacterium Vibrio sp. strain ANT-300. J Gen Microbiol. 1992 Oct;138(10):2075–2082. doi: 10.1099/00221287-138-10-2075. [DOI] [PubMed] [Google Scholar]
- Araki T. Changes in rates of synthesis of individual proteins in a psychrophilic bacterium after a shift in temperature. Can J Microbiol. 1991 Nov;37(11):840–847. doi: 10.1139/m91-145. [DOI] [PubMed] [Google Scholar]
- Araki T. The effect of temperature shifts on protein synthesis by the psychrophilic bacterium Vibrio sp. strain ANT-300. J Gen Microbiol. 1991 Apr;137(4):817–826. doi: 10.1099/00221287-137-4-817. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Pollitt N. S., Inouye M. Major cold shock protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(1):283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschal J. C. Some reflections on microbial competitiveness among heterotrophic bacteria. Antonie Van Leeuwenhoek. 1985;51(5-6):473–494. doi: 10.1007/BF00404494. [DOI] [PubMed] [Google Scholar]
- Harder W., Veldkamp H. Competition of marine psychrophilic bacteria at low temperatures. Antonie Van Leeuwenhoek. 1971;37(1):51–63. doi: 10.1007/BF02218466. [DOI] [PubMed] [Google Scholar]
- Jones P. G., VanBogelen R. A., Neidhardt F. C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987 May;169(5):2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrabee K. L., Phillips J. O., Williams G. J., Larrabee A. R. The relative rates of protein synthesis and degradation in a growing culture of Escherichia coli. J Biol Chem. 1980 May 10;255(9):4125–4130. [PubMed] [Google Scholar]
- Matin A., Veldkamp H. Physiological basis of the selective advantage of a Spirillum sp. in a carbon-limited environment. J Gen Microbiol. 1978 Apr;105(2):187–197. doi: 10.1099/00221287-105-2-187. [DOI] [PubMed] [Google Scholar]
- Nedwell D. B., Rutter M. Influence of temperature on growth rate and competition between two psychrotolerant Antarctic bacteria: low temperature diminishes affinity for substrate uptake. Appl Environ Microbiol. 1994 Jun;60(6):1984–1992. doi: 10.1128/aem.60.6.1984-1992.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell N. J. Cold adaptation of microorganisms. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1237):595-608, discussion 608-11. doi: 10.1098/rstb.1990.0034. [DOI] [PubMed] [Google Scholar]
- Taylor P. A., leB Williams P. J. Theoretical studies on the coexistence of competing species under continuous-flow conditions. Can J Microbiol. 1975 Jan;21(1):90–98. doi: 10.1139/m75-013. [DOI] [PubMed] [Google Scholar]
- Tilman D. Resource competition and community structure. Monogr Popul Biol. 1982;17:1–296. [PubMed] [Google Scholar]


