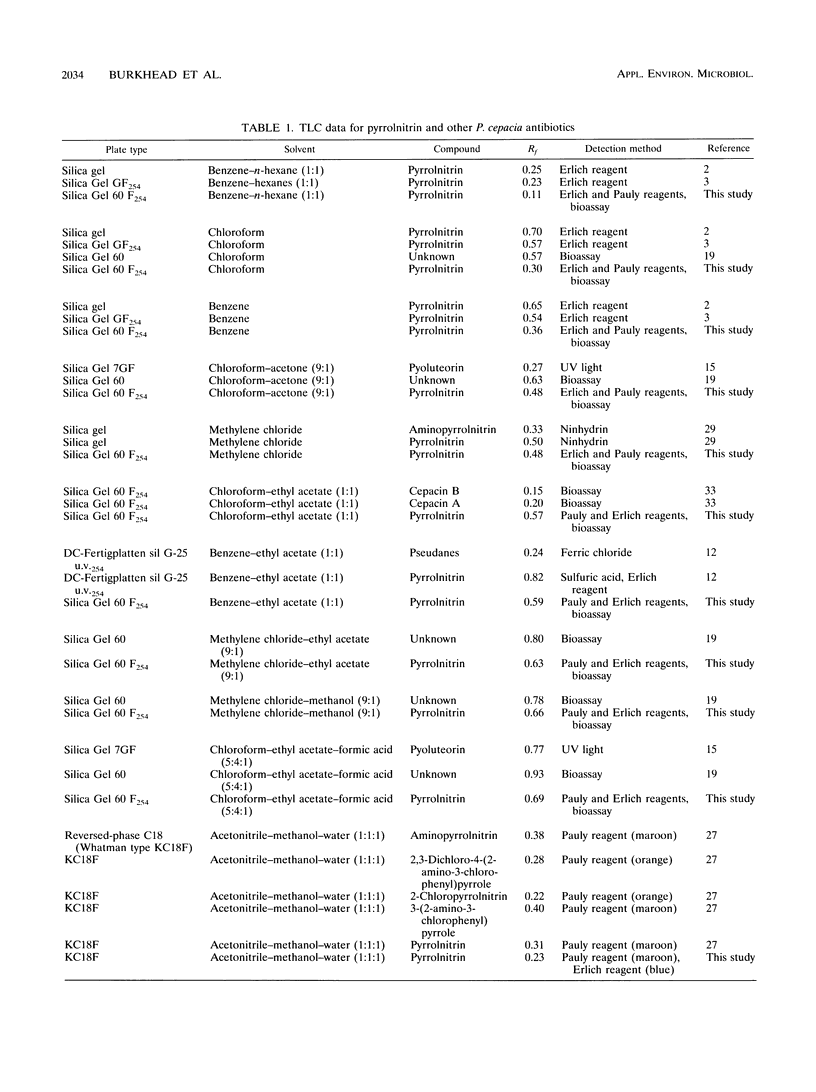
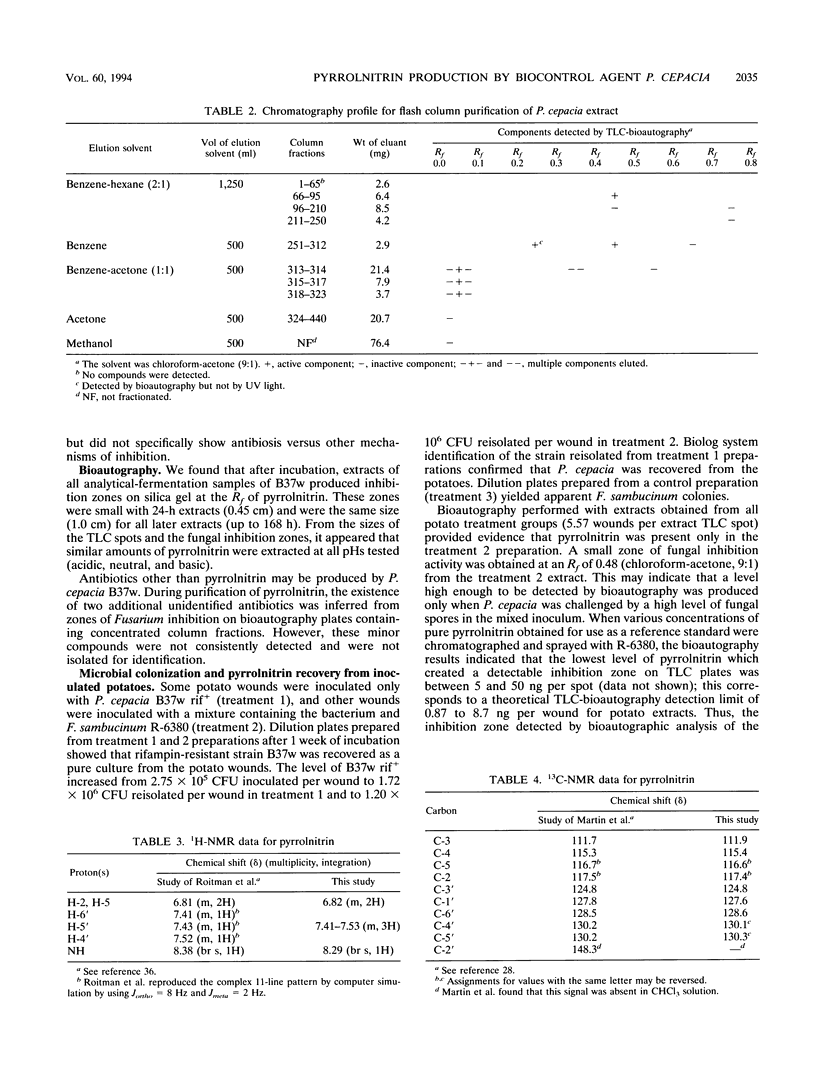
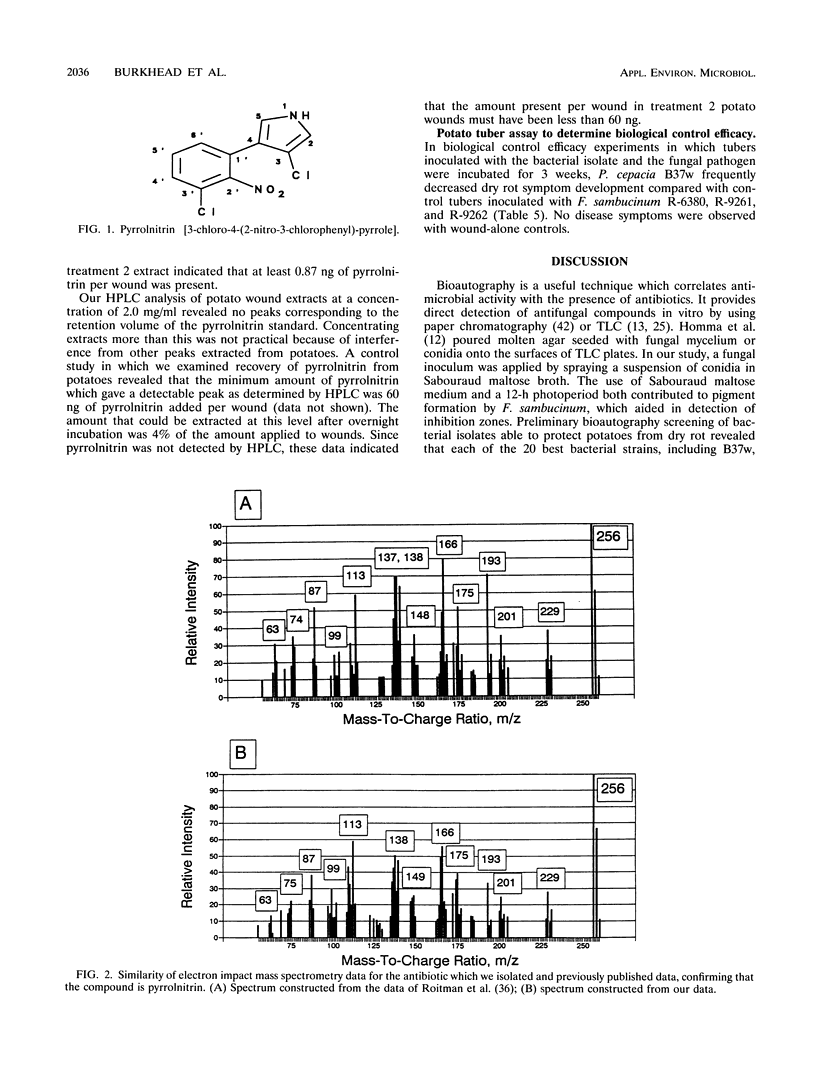
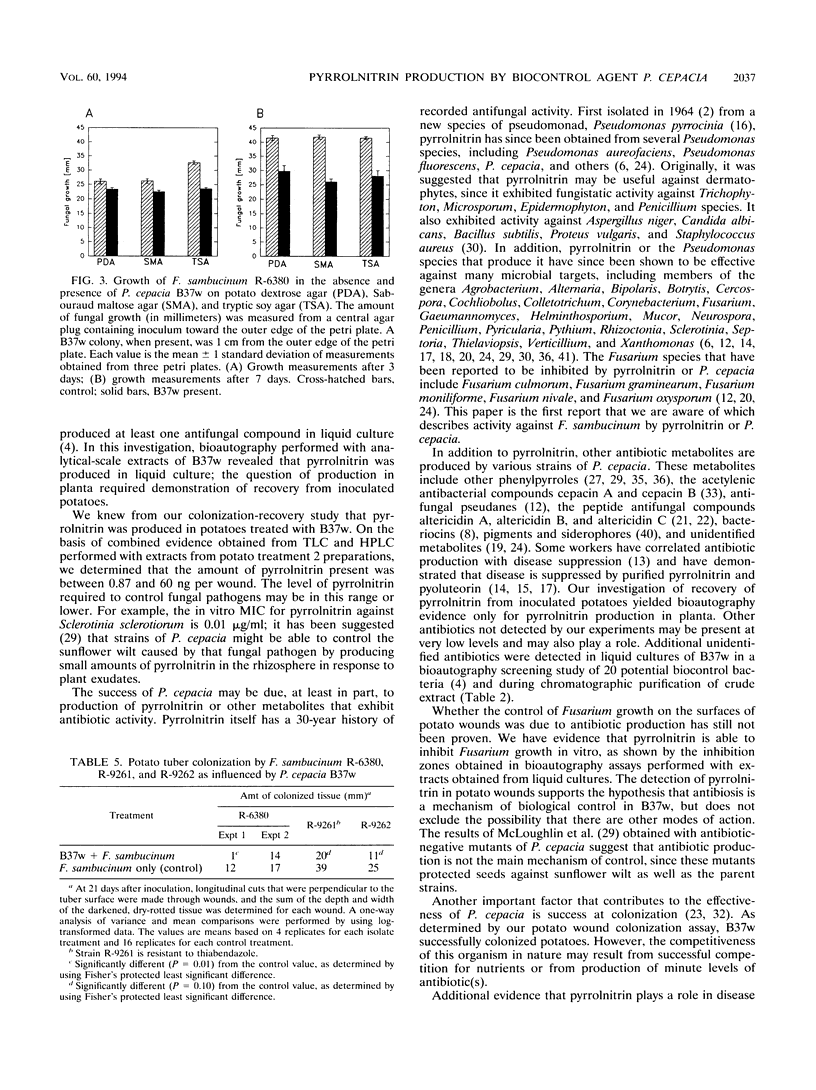
Abstract
Bacterial strain B37w (= NRRL B-14858), an isolate noteworthy because it inhibits the growth of the bioherbicide fungus Colletotrichum truncatum, was selected for further studies of bacterial antifungal properties. This isolate was identified as a Pseudomonas cepacia strain by performing carbohydrate utilization and fatty acid profile analyses, as well as other biochemical and physiological tests. Petri plate assays revealed that strain B37w exhibited antifungal activity against the potato dry rot fungus Fusarium sambucinum. Using bioautography, we correlated antifungal activity with production of a specific compound. Isolation from strain B37w and identification of the antifungal antibiotic pyrrolnitrin are described. A whole-potato assay revealed B37w's ability to colonize potato wounds. Wounded potatoes were inoculated with B37w, and pyrrolnitrin was detected in these potatoes by thin-layer chromatography-bioautography at a concentration on the order of nanograms per wound. We performed an assay in which we examined efficacy against F. sambucinum-incited potato dry rot and found that B37w inhibited disease development. This is the first report of P. cepacia or pyrrolnitrin activity against the economically important potato pathogen F. sambucinum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A 13C nuclear magnetic resonance study on the biosynthesis of pyrrolnitrin from tryptophan by Pseudomonas. J Am Chem Soc. 1972 Dec 13;94(25):8942–8944. doi: 10.1021/ja00780a070. [DOI] [PubMed] [Google Scholar]
- Arima K., Imanaka H., Kousaka M., Fukuda A., Tamura G. Studies on pyrrolnitrin, a new antibiotic. I. Isolation and properties of pyrrolnitrin. J Antibiot (Tokyo) 1965 Sep;18(5):201–204. [PubMed] [Google Scholar]
- Elander R. P., Mabe J. A., Hamill R. H., Gorman M. Metabolism of tryptophans by Pseudomonas aureofaciens. VI. Production of pyrrolnitrin by selected Pseudomonas species. Appl Microbiol. 1968 May;16(5):753–758. doi: 10.1128/am.16.5.753-758.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. F., Vidaver A. K. Bacteriocin, plasmid and pectolytic diversity in Pseudomonas cepacia of clinical and plant origin. J Gen Microbiol. 1979 Jan;110(1):161–170. doi: 10.1099/00221287-110-1-161. [DOI] [PubMed] [Google Scholar]
- Hill D. S., Stein J. I., Torkewitz N. R., Morse A. M., Howell C. R., Pachlatko J. P., Becker J. O., Ligon J. M. Cloning of Genes Involved in the Synthesis of Pyrrolnitrin from Pseudomonas fluorescens and Role of Pyrrolnitrin Synthesis in Biological Control of Plant Disease. Appl Environ Microbiol. 1994 Jan;60(1):78–85. doi: 10.1128/aem.60.1.78-85.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homans A. L., Fuchs A. Direct bioautography on thin-layer chromatograms as a method for detecting fungitoxic substances. J Chromatogr. 1970 Sep 16;51(2):327–329. doi: 10.1016/s0021-9673(01)96877-3. [DOI] [PubMed] [Google Scholar]
- Jayaswal R. K., Fernandez M. A., Schroeder R. G. Isolation and characterization of a pseudomonas strain that restricts growth of various phytopathogenic fungi. Appl Environ Microbiol. 1990 Apr;56(4):1053–1058. doi: 10.1128/aem.56.4.1053-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert B., Leyns F., Van Rooyen L., Gosselé F., Papon Y., Swings J. Rhizobacteria of maize and their antifungal activities. Appl Environ Microbiol. 1987 Aug;53(8):1866–1871. doi: 10.1128/aem.53.8.1866-1871.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin T. J., Quinn J. P., Bettermann A., Bookland R. Pseudomonas cepacia suppression of sunflower wilt fungus and role of antifungal compounds in controlling the disease. Appl Environ Microbiol. 1992 May;58(5):1760–1763. doi: 10.1128/aem.58.5.1760-1763.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Watanabe N. Pyrrolnitrin, a new antifungal antibiotic. Microbiological and toxicological observations. J Antibiot (Tokyo) 1965 Sep;18(5):211–219. [PubMed] [Google Scholar]
- Parker W. L., Rathnum M. L., Seiner V., Trejo W. H., Principe P. A., Sykes R. B. Cepacin A and cepacin B, two new antibiotics produced by Pseudomonas cepacia. J Antibiot (Tokyo) 1984 May;37(5):431–440. doi: 10.7164/antibiotics.37.431. [DOI] [PubMed] [Google Scholar]


