Abstract
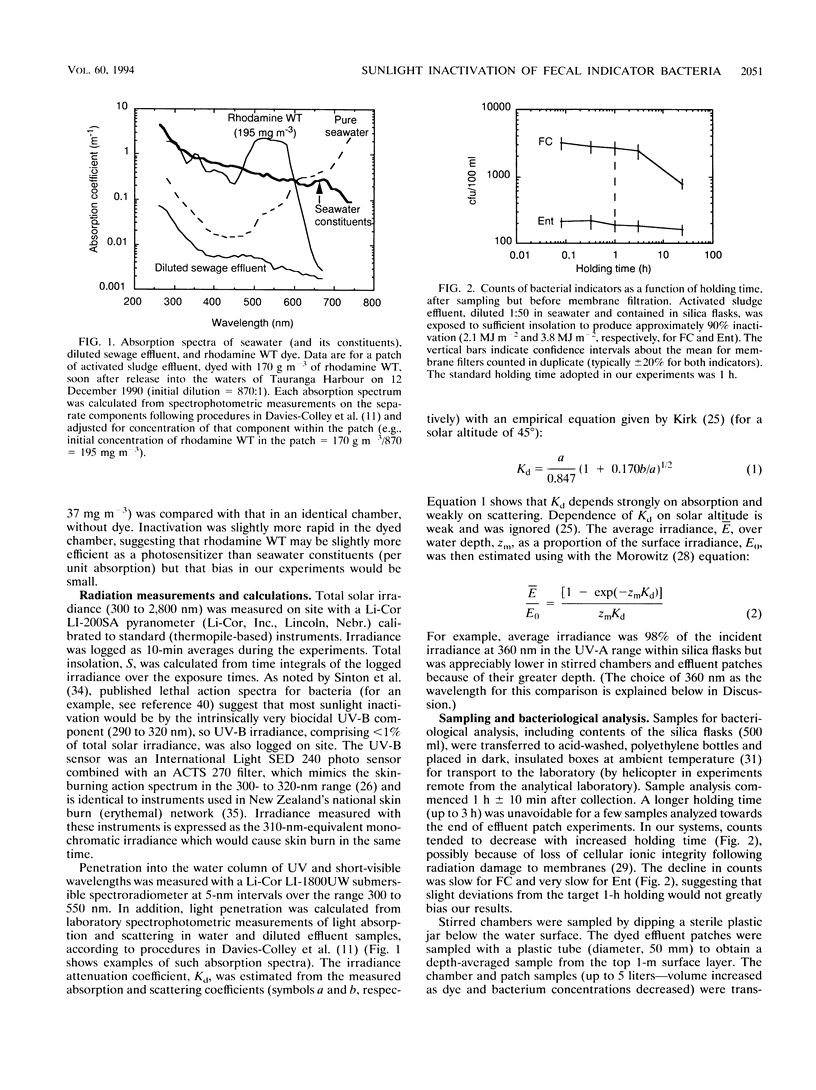
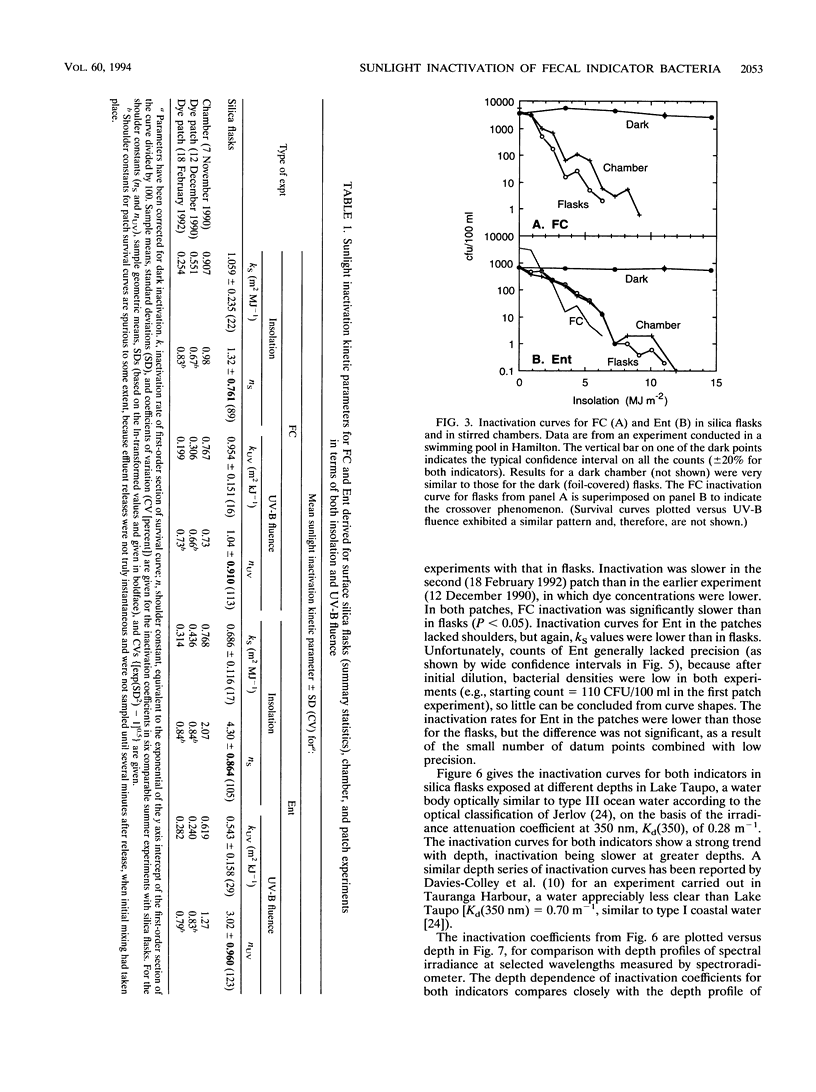
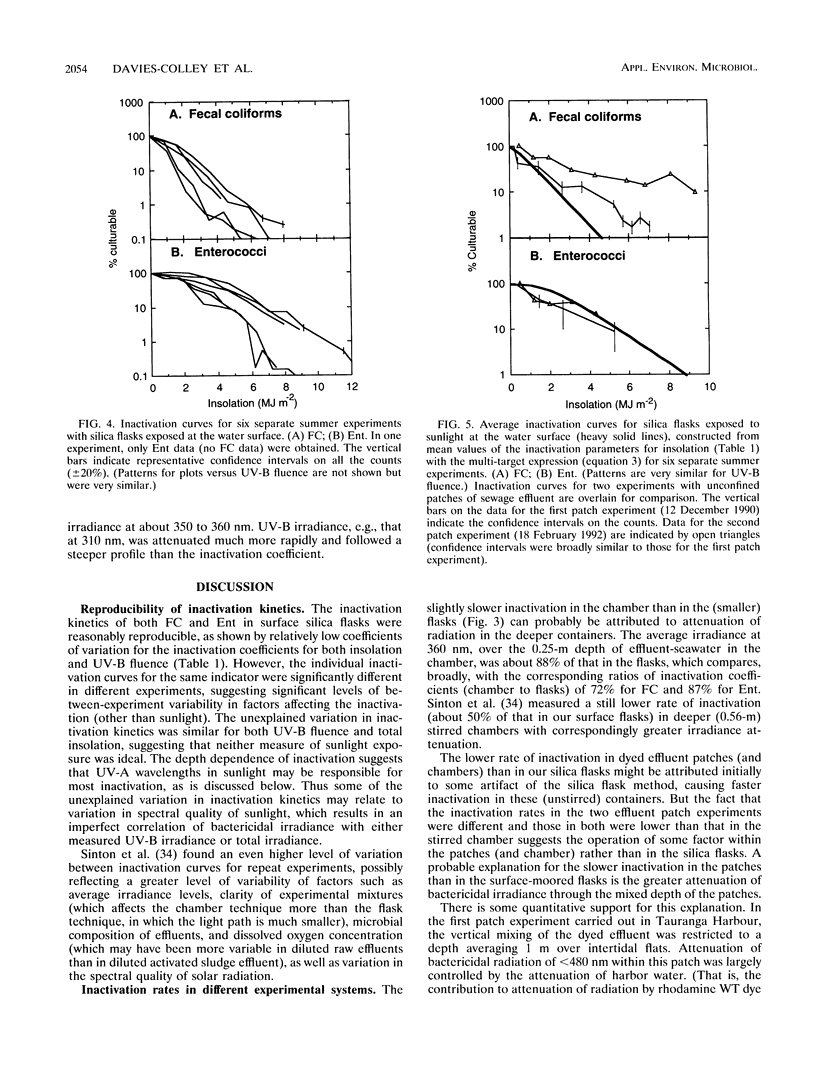
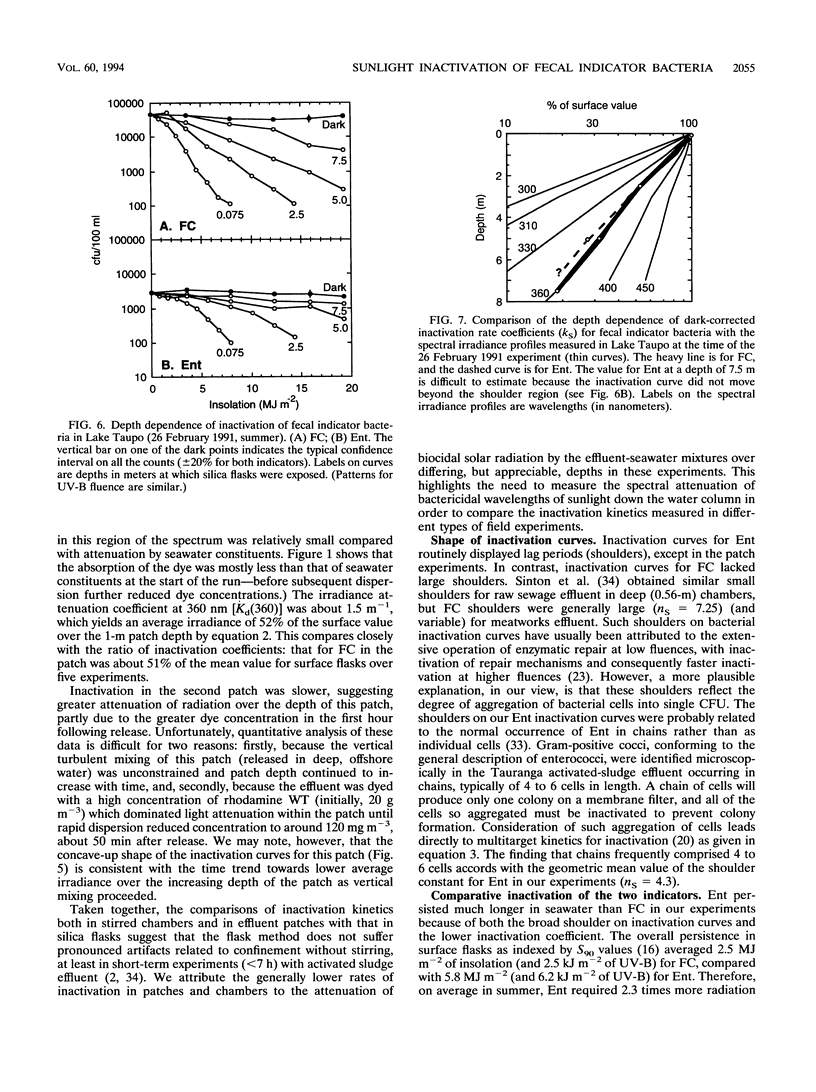
Inactivation (loss of culturability) by sunlight of enterococci and fecal coliforms within sewage effluent diluted in seawater was investigated in field experiments. In most experiments, 500-ml flasks of pure silica were used to confine activated sludge effluent diluted to 2% (vol/vol) in seawater. Inactivation of bacteria in these flasks (diameter, 0.1 m) was faster than in either open chambers (depth, 0.25 m) or patches of dyed effluent (depth of order, 1 m), probably because of the longer light paths in the latter two types of experiment, which caused greater attenuation of sunlight. Inactivation of 90% of enterococci generally required 2.3 times the insolation required for 90% inactivation of fecal coliforms, because of both the presence of larger initial shoulders on survival curves and a lower final inactivation rate. Two parameters are required to model inactivation of enterococci, a shoulder constant as well as a rate coefficient. The depth dependence of inactivation rate for both fecal indicators matched the attenuation profile of UV-A radiation at about 360 nm. Inactivation by UV-B radiation (290 to 320 nm), which penetrates much less into seawater, is of minor importance compared with the UV-A and visible radiation in sunlight, contrary to expectations in consideration of published action spectra for bacterial inactivation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabelli V. J., Dufour A. P., McCabe L. J., Levin M. A. Swimming-associated gastroenteritis and water quality. Am J Epidemiol. 1982 Apr;115(4):606–616. doi: 10.1093/oxfordjournals.aje.a113342. [DOI] [PubMed] [Google Scholar]
- Curtis T. P., Mara D. D., Silva S. A. Influence of pH, Oxygen, and Humic Substances on Ability of Sunlight To Damage Fecal Coliforms in Waste Stabilization Pond Water. Appl Environ Microbiol. 1992 Apr;58(4):1335–1343. doi: 10.1128/aem.58.4.1335-1343.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnison A. M., Ross C. M., Russell J. M. Quality control of bacterial enumeration. Appl Environ Microbiol. 1993 Mar;59(3):922–923. doi: 10.1128/aem.59.3.922-923.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka R. S., Hashimoto H. H., Siwak E. B., Young R. H. Effect of sunlight on survival of indicator bacteria in seawater. Appl Environ Microbiol. 1981 Mar;41(3):690–696. doi: 10.1128/aem.41.3.690-696.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar A. H., During M. Evaluation of the Anderson Baird-Parker direct plating method for enumerating Escherichia coli in water. J Appl Bacteriol. 1988 Jan;64(1):89–98. doi: 10.1111/j.1365-2672.1988.tb02432.x. [DOI] [PubMed] [Google Scholar]
- MOROWITZ H. J. Absorption effects in volume irradiation of microorganisms. Science. 1950 Mar 3;111(2879):229–229. doi: 10.1126/science.111.2879.229-a. [DOI] [PubMed] [Google Scholar]
- Moss S. H., Smith K. C. Membrane damage can be a significant factor in the inactivation of Escherichia coli by near-ultraviolet radiation. Photochem Photobiol. 1981 Feb;33(2):203–210. doi: 10.1111/j.1751-1097.1981.tb05325.x. [DOI] [PubMed] [Google Scholar]
- Pagel J. E., Qureshi A. A., Young D. M., Vlassoff L. T. Comparison of four membrane filter methods for fecal coliform enumeration. Appl Environ Microbiol. 1982 Apr;43(4):787–793. doi: 10.1128/aem.43.4.787-793.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinton L. W., Davies-Colley R. J., Bell R. G. Inactivation of enterococci and fecal coliforms from sewage and meatworks effluents in seawater chambers. Appl Environ Microbiol. 1994 Jun;60(6):2040–2048. doi: 10.1128/aem.60.6.2040-2048.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R. B., Brown M. S. Action spectra for oxygen-dependent and independent inactivation of Escherichia coli WP2s from 254 to 460 nm. Photochem Photobiol. 1979 Feb;29(2):407–409. doi: 10.1111/j.1751-1097.1979.tb07068.x. [DOI] [PubMed] [Google Scholar]
- Webb R. B., Brown M. S. Sensitivity of strains of Escherichia coli differing in repair capability to far UV, near UV and visible radiations. Photochem Photobiol. 1976 Nov;24(5):425–432. doi: 10.1111/j.1751-1097.1976.tb06849.x. [DOI] [PubMed] [Google Scholar]


