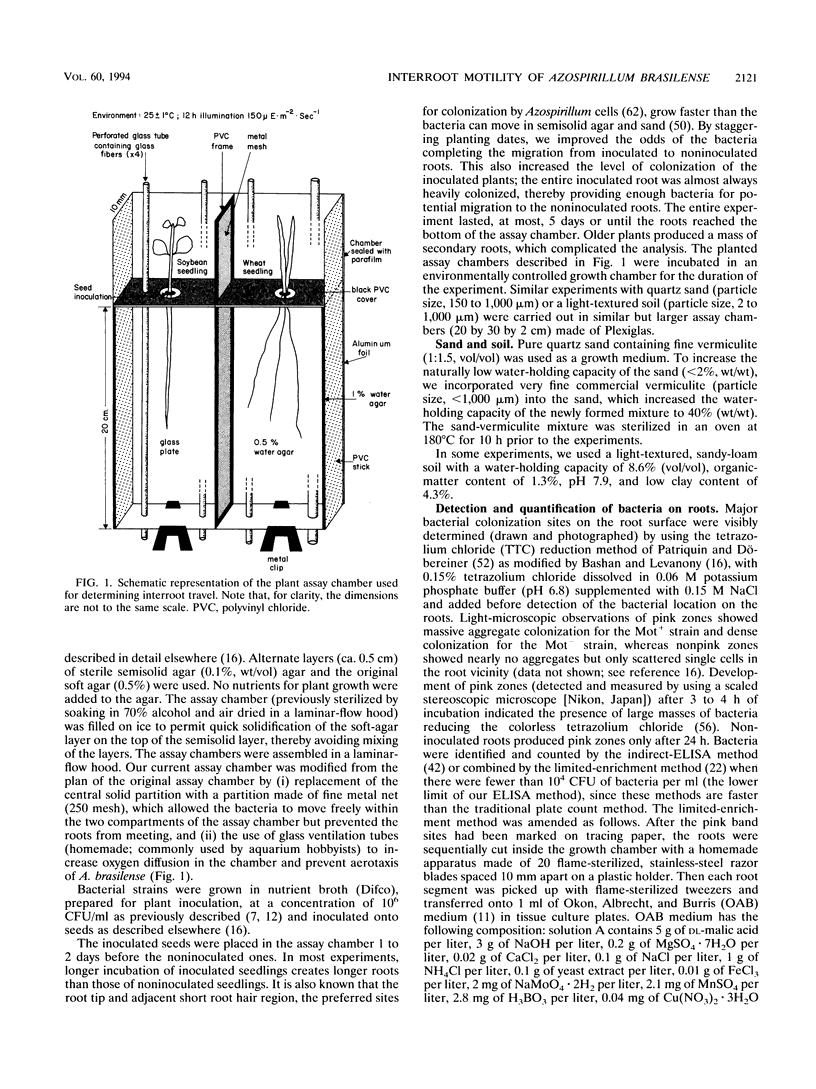
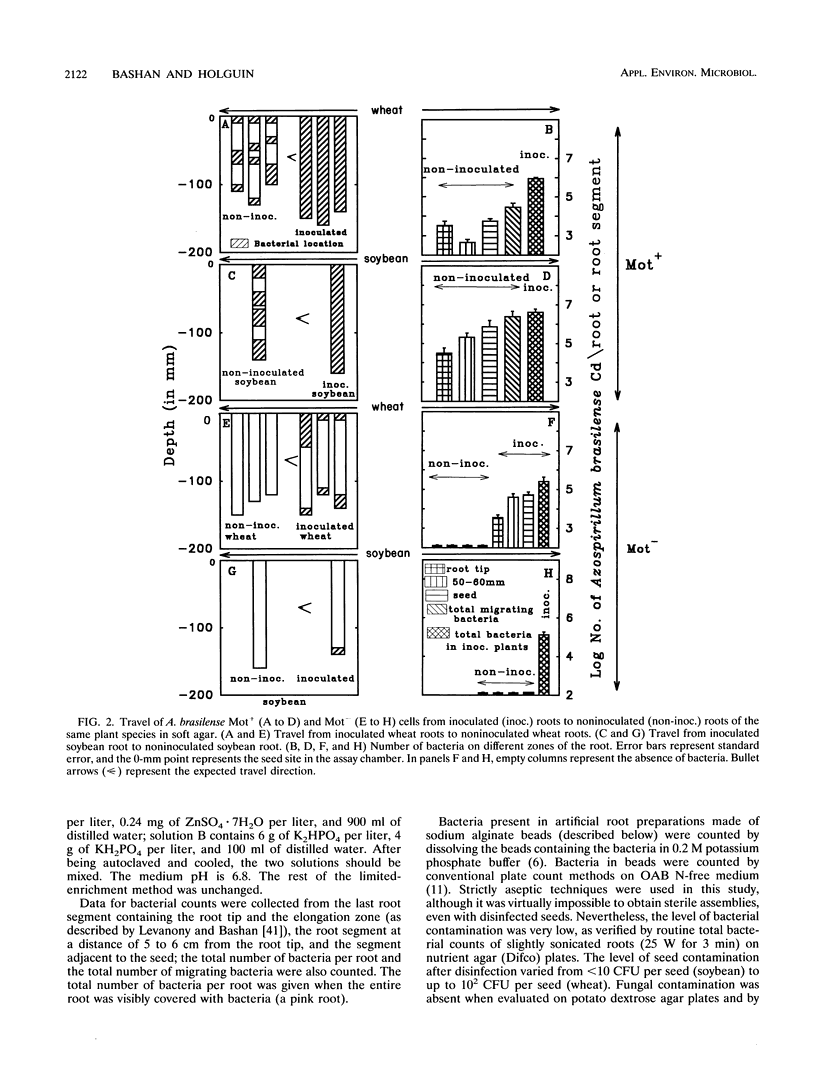
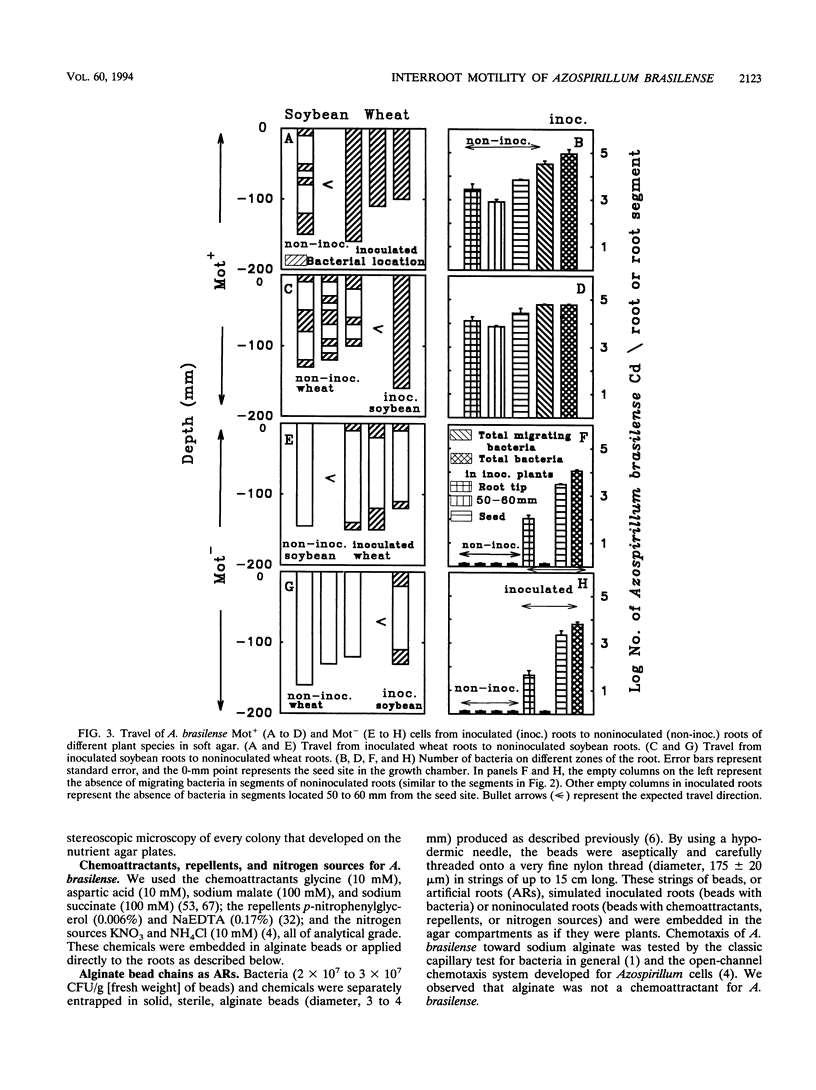
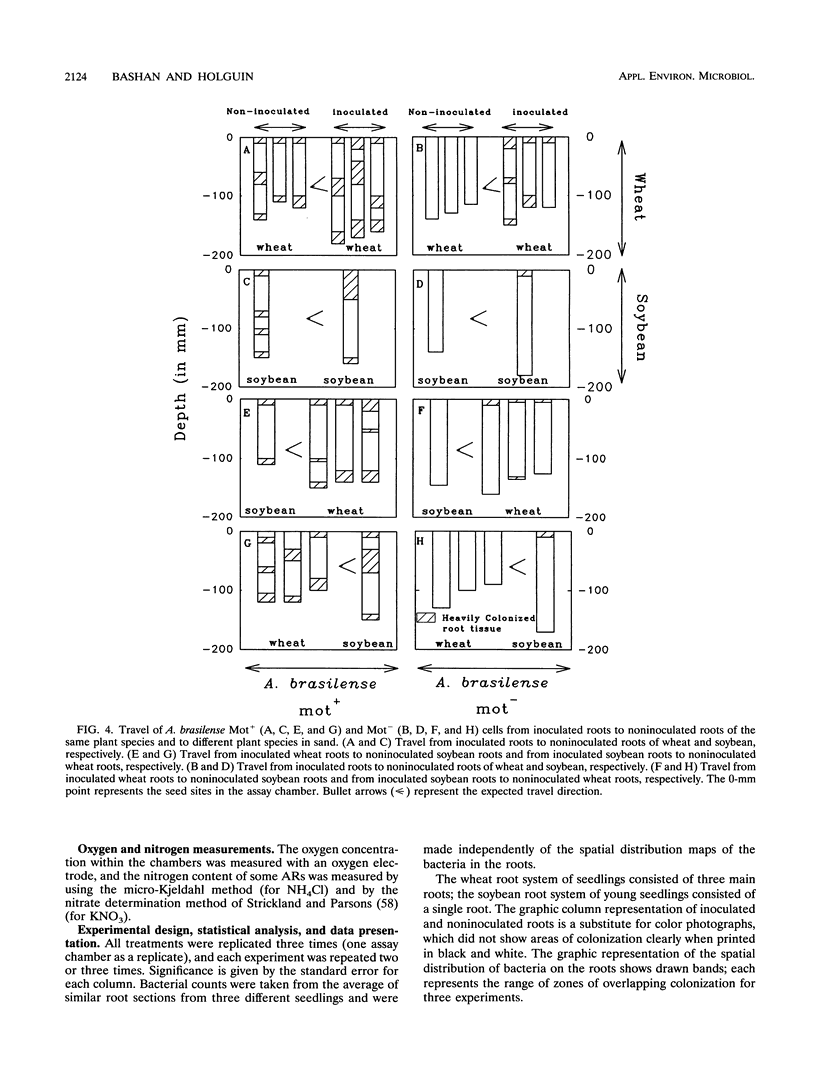
Abstract
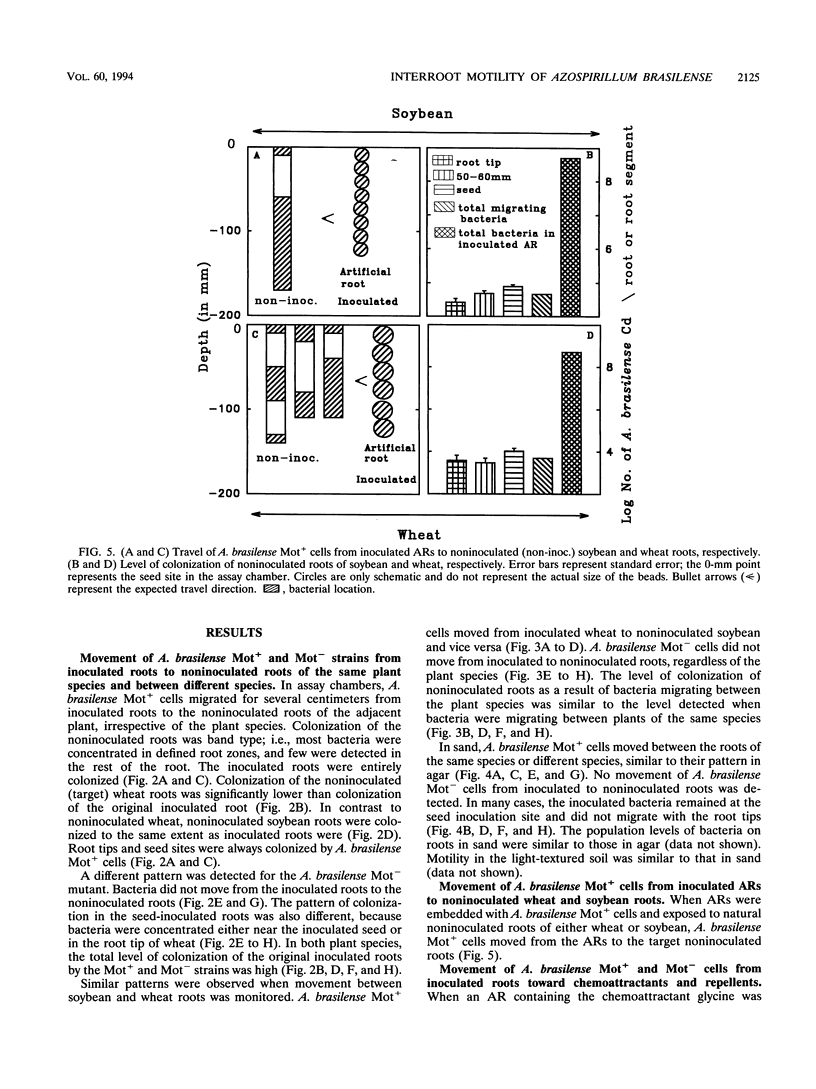
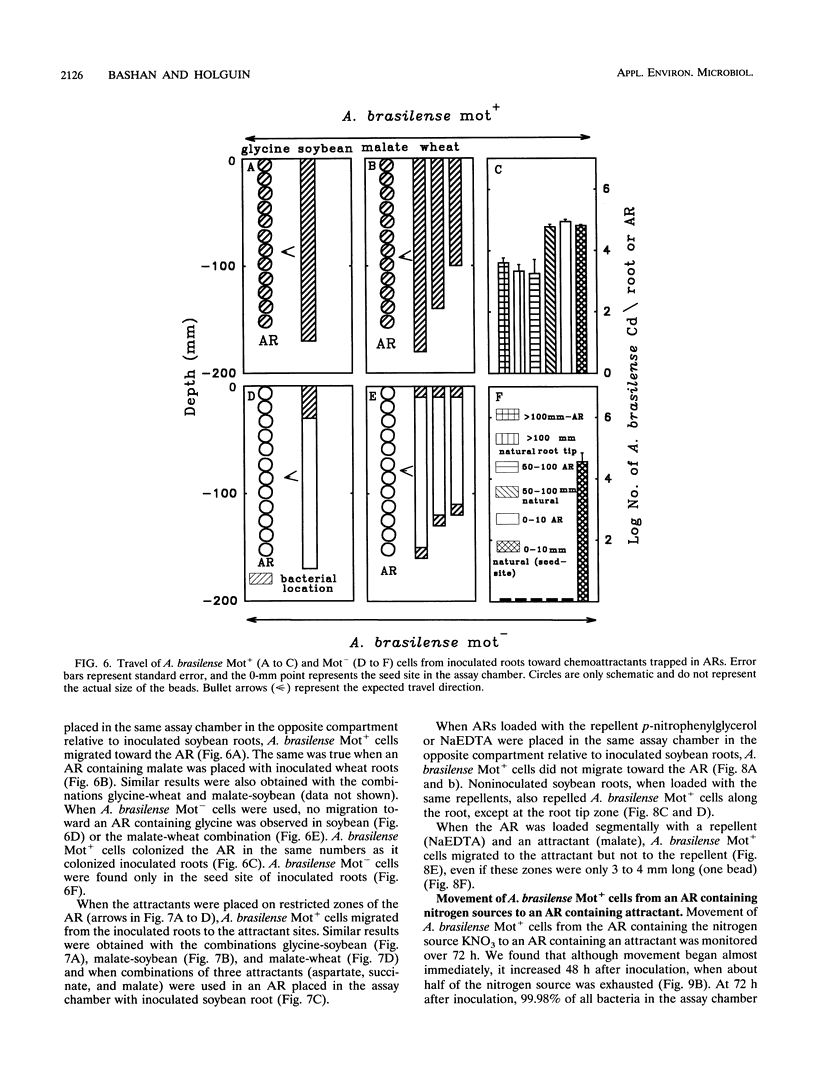
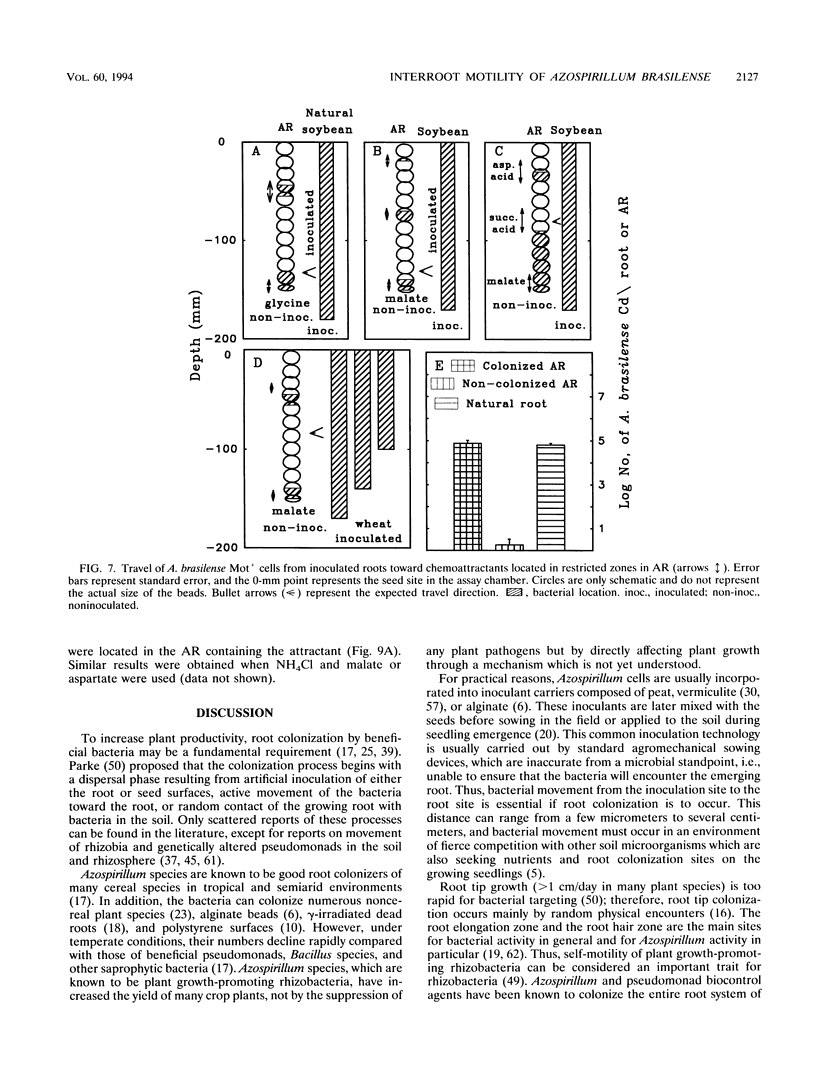
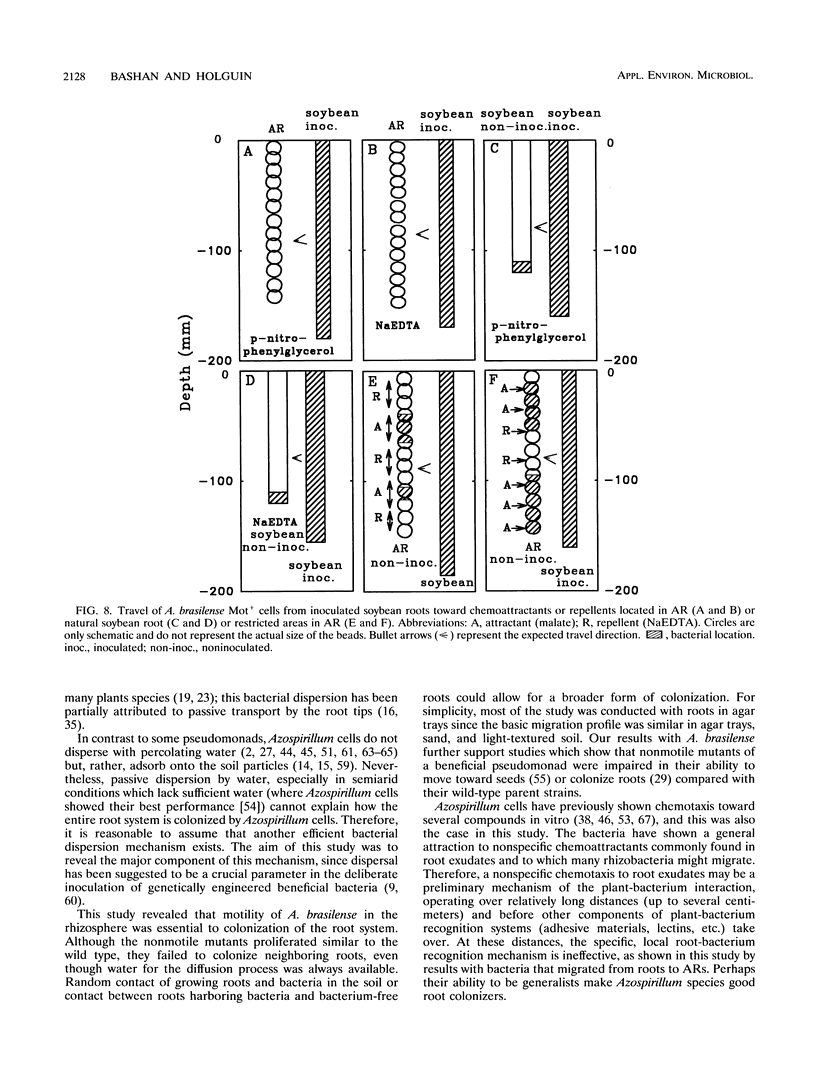
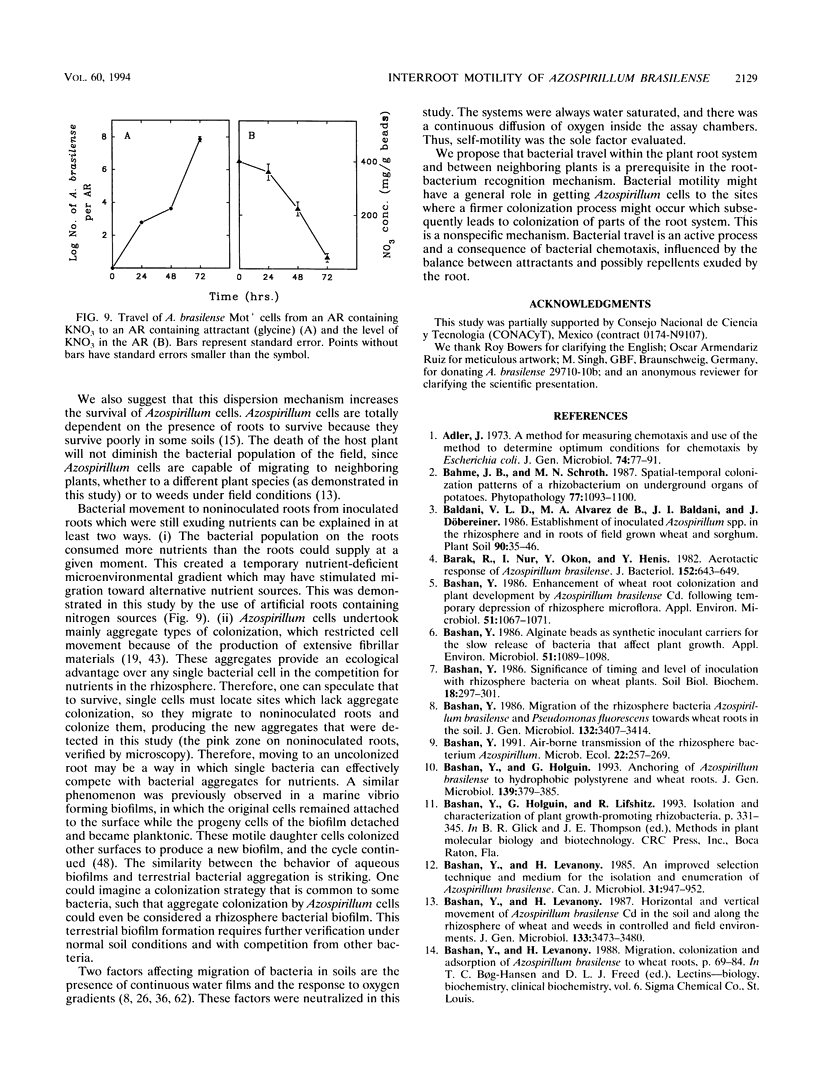
The root-to-root travel of the beneficial bacterium Azospirillum brasilense on wheat and soybean roots in agar, sand, and light-textured soil was monitored. We used a motile wild-type (Mot+) strain and a motility-deficient (Mot-) strain which was derived from the wild-type strain. The colonization levels of inoculated roots were similar for the two strains. Mot+ cells moved from inoculated roots (either natural or artificial roots in agar, sand, or light-textured soil) to noninoculated roots, where they formed a band-type colonization composed of bacterial aggregates encircling a limited part of the root, regardless of the plant species. The Mot- strain did not move toward noninoculated roots of either plant species and usually stayed at the inoculation site and root tips. The effect of attractants and repellents was the primary factor governing the motility of Mot+ cells in the presence of adequate water. We propose that interroot travel of A. brasilense is an essential preliminary step in the root-bacterium recognition mechanism. Bacterial motility might have a general role in getting Azospirillum cells to the site where firmer attachment favors colonization of the root system. Azospirillum travel toward plants is a nonspecific active process which is not directly dependent on nutrient deficiency but is a consequence of a nonspecific bacterial chemotaxis, influenced by the balance between attractants and possibly repellents leaked by the root.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Barak R., Nur I., Okon Y., Henis Y. Aerotactic response of Azospirillum brasilense. J Bacteriol. 1982 Nov;152(2):643–649. doi: 10.1128/jb.152.2.643-649.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan Y. Alginate beads as synthetic inoculant carriers for slow release of bacteria that affect plant growth. Appl Environ Microbiol. 1986 May;51(5):1089–1098. doi: 10.1128/aem.51.5.1089-1098.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan Y. Enhancement of Wheat Root Colonization and Plant Development by Azospirillum brasilense Cd. Following Temporary Depression of Rhizosphere Microflora. Appl Environ Microbiol. 1986 May;51(5):1067–1071. doi: 10.1128/aem.51.5.1067-1071.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weger L. A., van der Vlugt C. I., Wijfjes A. H., Bakker P. A., Schippers B., Lugtenberg B. Flagella of a plant-growth-stimulating Pseudomonas fluorescens strain are required for colonization of potato roots. J Bacteriol. 1987 Jun;169(6):2769–2773. doi: 10.1128/jb.169.6.2769-2773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz P., Süssmuth R. A highly sensitive bacterial assay for toxins based on swarming inhibition, and comparison with the cup plate assay based on growth inhibition. Toxicology. 1987 Aug;45(2):185–191. doi: 10.1016/0300-483x(87)90104-1. [DOI] [PubMed] [Google Scholar]
- Patriquin D. G., Döbereiner J. Light microscopy observations of tetrazolium-reducing bacteria in the endorhizosphere of maize and other grasses in Brazil. Can J Microbiol. 1978 Jun;24(6):734–742. doi: 10.1139/m78-122. [DOI] [PubMed] [Google Scholar]
- Reinhold B., Hurek T., Fendrik I. Strain-specific chemotaxis of Azospirillum spp. J Bacteriol. 1985 Apr;162(1):190–195. doi: 10.1128/jb.162.1.190-195.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH F. E. Tetrazolium salt. Science. 1951 Jun 29;113(2948):751–754. doi: 10.1126/science.113.2948.751-a. [DOI] [PubMed] [Google Scholar]
- Trevors J. T., van Elsas J. D., van Overbeek L. S., Starodub M. E. Transport of a genetically engineered Pseudomonas fluorescens strain through a soil microcosm. Appl Environ Microbiol. 1990 Feb;56(2):401–408. doi: 10.1128/aem.56.2.401-408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhulin I. B., Armitage J. P. Motility, chemokinesis, and methylation-independent chemotaxis in Azospirillum brasilense. J Bacteriol. 1993 Feb;175(4):952–958. doi: 10.1128/jb.175.4.952-958.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


