Abstract
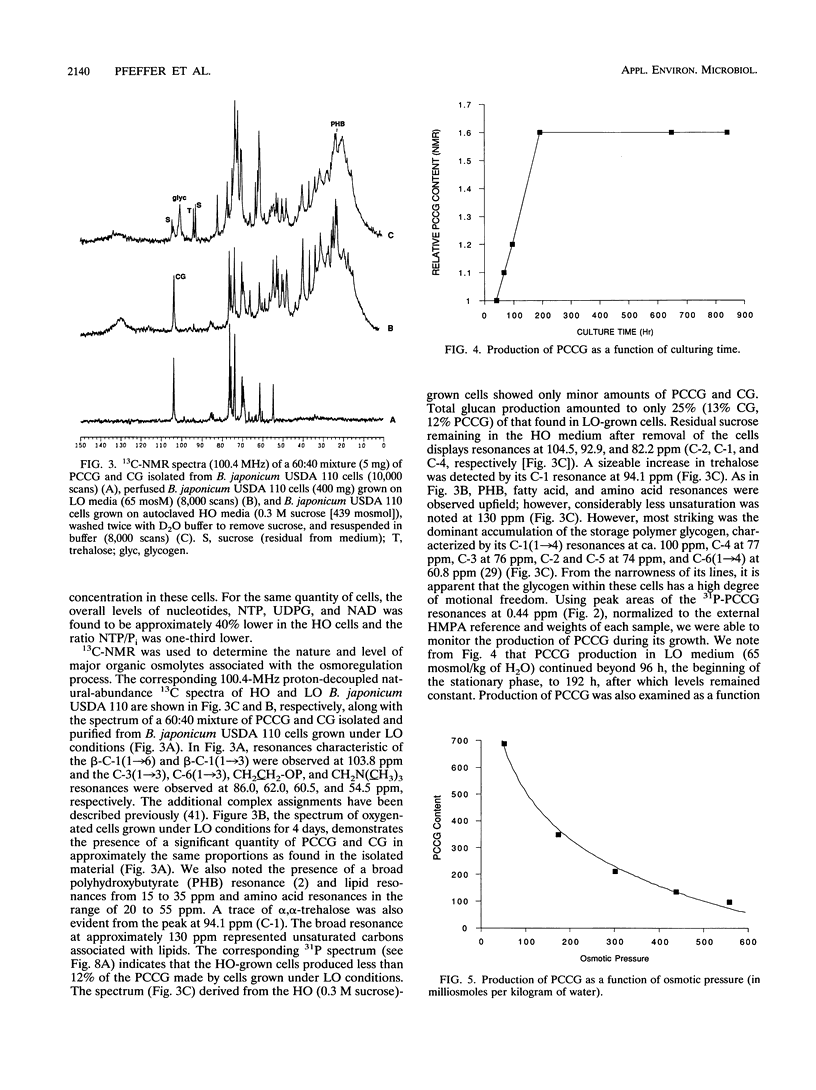
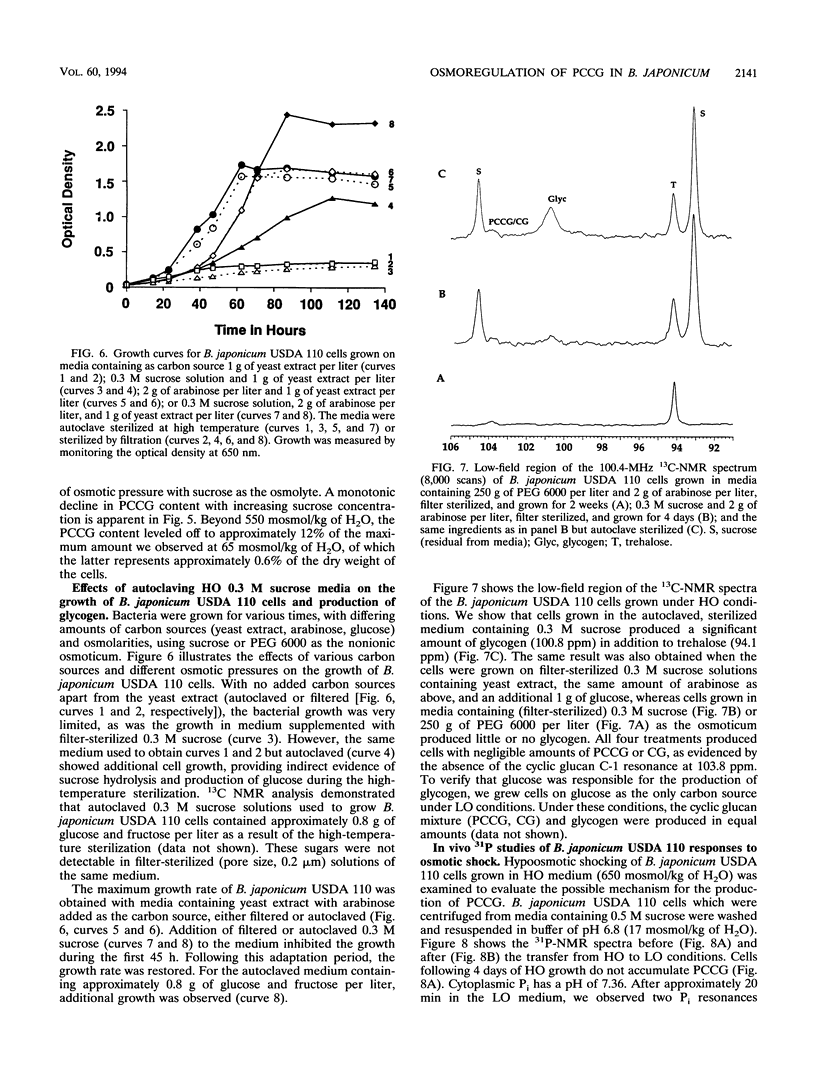
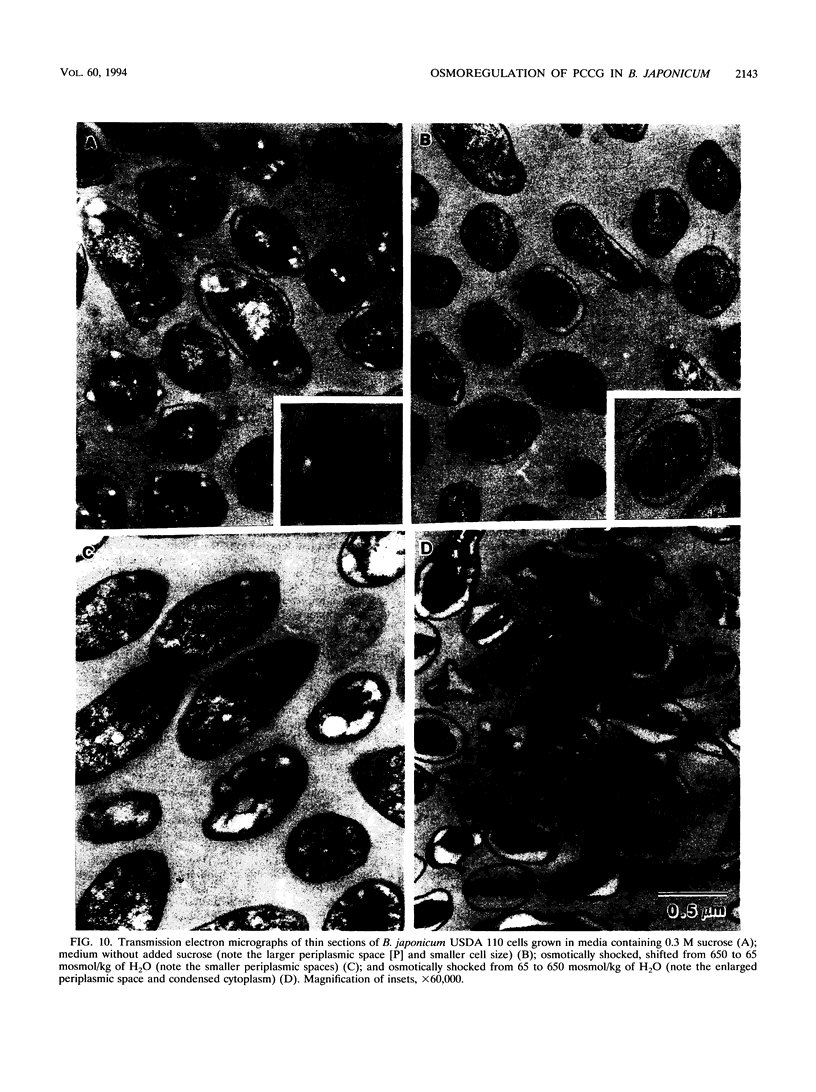
A phosphocholine-substituted beta-1,3;1,6 cyclic glucan (PCCG), an unusual cyclic oligosaccharide, has been isolated from Bradyrhizobium japonicum USDA 110 (D. B. Rolin, P. E. Pfeffer, S. F. Osman, B. S. Swergold, F. Kappler, and A. J. Benesi, Biochim. Biophys. Acta 1116:215-225, 1992). Data presented here suggest that PCCG synthesis is dependent on the carbon metabolism and that osmotic regulation of its biosynthesis parallels regulation of membrane-derived oligosaccharide biosynthesis observed in Escherichia coli (E. P. Kennedy, M. K. Rumley, H. Schulman, and L. M. G. van Golde, J. Biol. Chem. 251:4208-4213, 1976) and Agrobacterium tumefaciens (G. A. Cangelosi, G. Martinetti, and E. W. Nester, J. Bacteriol. 172:2172-2174, 1990). Growth of B. japonicum USDA 110 cells in the reference medium at relatively low osmotic pressures (LO) (65 mosmol/kg of H2O) caused a large accumulation of PCCG and unsubstituted beta-1,3;1,6 cyclic glucans (CG). Sucrose and polyethylene glycol, nonionic osmotica, reduce all growth rates and inhibit almost completely the production of PCCG at high osmotic pressures (HO) above 650 and 400 mosmol/kg of H2O), respectively. We used in vivo 13C nuclear magnetic resonance spectroscopy to identify the active osmolytes implicated in the osmoregulation process. The level of alpha,alpha-trehalose in B. japonicum cells grown in autoclaved or filter-sterilized solutions remained constant in HO (0.3 M sucrose or 250 g of polyethylene glycol 6000 per liter) medium. Significant amounts of glycogen and extracellular polysaccharides were produced only when glucose was present in the autoclaved HO 0.3 M sucrose media. The results of hypo- and hyperosmotic shocking of B. japonicum USDA 110 cells were monitored by using in vivo 31P and 13C nuclear magnetic resonance spectroscopy. The first observed osmoregulatory response of glycogen-containing cells undergoing hypoosmotic shock was release of P(i) into the medium. Within 7 h, reabsorption of P(i) was complete and production of PCCG was initiated. After 12 h, the PCCG content had increased by a factor of 7. Following the same treatment, cells containing little or no glycogen released trehalose and failed to produce PCCG. Thus the production of PCCG/CG in response to hypoosmotic shocking of stationary-phase cells was found to be directly linked to the interconversion of stored glycogen. Hyperosmotic shocking of LO-grown stationary-phase cells with sucrose had no effect on the content of previously synthesized CG/PCCG. The PCCG/CG content and its osmotically induced biosynthesis are discussed in terms of carbon metabolism and a possible role in hypoosmotic adaptation in B. japonicum USDA 110.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard G. N., Sanders J. K. The poly-beta-hydroxybutyrate granule in vivo. A new insight based on NMR spectroscopy of whole cells. J Biol Chem. 1989 Feb 25;264(6):3286–3291. [PubMed] [Google Scholar]
- Bhagwat A. A., Tully R. E., Keister D. L. Identification and cloning of a cyclic beta-(1-->3), beta-(1-->6)-D-glucan synthesis locus from Bradyrhizobium japonicum. FEMS Microbiol Lett. 1993 Dec 1;114(2):139–144. doi: 10.1111/j.1574-6968.1993.tb06564.x. [DOI] [PubMed] [Google Scholar]
- Breedveld M. W., Zevenhuizen L. P., Zehnder A. J. Synthesis of cyclic beta-(1,2)-glucans by Rhizobium leguminosarum biovar trifolii TA-1: factors influencing excretion. J Bacteriol. 1992 Oct;174(20):6336–6342. doi: 10.1128/jb.174.20.6336-6342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangelosi G. A., Martinetti G., Nester E. W. Osmosensitivity phenotypes of Agrobacterium tumefaciens mutants that lack periplasmic beta-1,2-glucan. J Bacteriol. 1990 Apr;172(4):2172–2174. doi: 10.1128/jb.172.4.2172-2174.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. L., Miller K. J. A novel membrane-bound glucosyltransferase from Bradyrhizobium japonicum. J Bacteriol. 1991 Jul;173(14):4271–4276. doi: 10.1128/jb.173.14.4271-4276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984 Feb 17;223(4637):701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Csonka L. N., Hanson A. D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnbier U., Limpinsel E., Schmid R., Bakker E. P. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch Microbiol. 1988;150(4):348–357. doi: 10.1007/BF00408306. [DOI] [PubMed] [Google Scholar]
- Dylan T., Helinski D. R., Ditta G. S. Hypoosmotic adaptation in Rhizobium meliloti requires beta-(1----2)-glucan. J Bacteriol. 1990 Mar;172(3):1400–1408. doi: 10.1128/jb.172.3.1400-1408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger O., Russo F. D., Silhavy T. J., Kennedy E. P. Membrane-derived oligosaccharides affect porin osmoregulation only in media of low ionic strength. J Bacteriol. 1992 Feb;174(4):1410–1413. doi: 10.1128/jb.174.4.1410-1413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger O., Weissborn A. C., Kennedy E. P. Biosynthesis and excretion of cyclic glucans by Rhizobium meliloti 1021. J Bacteriol. 1991 May;173(9):3021–3024. doi: 10.1128/jb.173.9.3021-3024.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia R. A., Cavaignac S., Zorreguieta A., Toro N., Olivares J., Ugalde R. A. A Rhizobium meliloti mutant that forms ineffective pseudonodules in alfalfa produces exopolysaccharide but fails to form beta-(1----2) glucan. J Bacteriol. 1987 Feb;169(2):880–884. doi: 10.1128/jb.169.2.880-884.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore R. S., Miller K. J. Cyclic [beta]-1,6 -1,3 Glucans Are Synthesized by Bradyrhizobium japonicum Bacteroids within Soybean (Glycine max) Root Nodules. Plant Physiol. 1993 May;102(1):191–194. doi: 10.1104/pp.102.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Signal exchange in plant-microbe interactions. Microbiol Rev. 1986 Jun;50(2):193–225. doi: 10.1128/mr.50.2.193-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B. J., Kennedy E. P. The biosynthesis of membrane-derived oligosaccharides. A membrane-bound phosphoglycerol transferase. J Biol Chem. 1983 Feb 25;258(4):2394–2398. [PubMed] [Google Scholar]
- Karl D. M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol Rev. 1980 Dec;44(4):739–796. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K. Osmotic regulation of biosynthesis of membrane-derived oligosaccharides in Escherichia coli. J Bacteriol. 1988 Jun;170(6):2457–2461. doi: 10.1128/jb.170.6.2457-2461.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K., Schulman H., Van Golde L. M. Identification of sn-glycero-1-phosphate and phosphoethanolamine residues linked to the membrane-derived Oligosaccharides of Escherichia coli. J Biol Chem. 1976 Jul 25;251(14):4208–4213. [PubMed] [Google Scholar]
- Miller K. J., Gore R. S., Benesi A. J. Phosphoglycerol substituents present on the cyclic beta-1,2-glucans of Rhizobium meliloti 1021 are derived from phosphatidylglycerol. J Bacteriol. 1988 Oct;170(10):4569–4575. doi: 10.1128/jb.170.10.4569-4575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Gore R. S., Johnson R., Benesi A. J., Reinhold V. N. Cell-associated oligosaccharides of Bradyrhizobium spp. J Bacteriol. 1990 Jan;172(1):136–142. doi: 10.1128/jb.172.1.136-142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Hadley J. A., Gustine D. L. Cyclic [beta]-1,6-1,3-Glucans of Bradyrhizobium japonicum USDA 110 Elicit Isoflavonoid Production in the Soybean (Glycine max) Host. Plant Physiol. 1994 Mar;104(3):917–923. doi: 10.1104/pp.104.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Reinhold V. N., Weissborn A. C., Kennedy E. P. Cyclic glucans produced by Agrobacterium tumefaciens are substituted with sn-1-phosphoglycerol residues. Biochim Biophys Acta. 1987 Jul 10;901(1):112–118. doi: 10.1016/0005-2736(87)90262-8. [DOI] [PubMed] [Google Scholar]
- Money N. P. Osmotic Pressure of Aqueous Polyethylene Glycols : Relationship between Molecular Weight and Vapor Pressure Deficit. Plant Physiol. 1989 Oct;91(2):766–769. doi: 10.1104/pp.91.2.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer P. E., Rolin D. B., Kumosinski T. F., Macfall J. S., Schmidt J. H. P relaxation responses associated with n(2)/o(2) diffusion in soybean nodule cortical cells and excised cortical tissue. Plant Physiol. 1992 Dec;100(4):1682–1690. doi: 10.1104/pp.100.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer P. E., Tu S. I., Gerasimowicz W. V., Cavanaugh J. R. In VivoP NMR Studies of Corn Root Tissue and Its Uptake of Toxic Metals. Plant Physiol. 1986 Jan;80(1):77–84. doi: 10.1104/pp.80.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolin D. B., Boswell R. T., Sloger C., Tu S. I., Pfeffer P. E. In VivoP NMR Spectroscopic Studies of Soybean Bradyrhizobium Symbiosis: I. Optimization of Parameters. Plant Physiol. 1989 Apr;89(4):1238–1246. doi: 10.1104/pp.89.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolin D. B., Pfeffer P. E., Osman S. F., Szwergold B. S., Kappler F., Benesi A. J. Structural studies of a phosphocholine substituted beta-(1,3);(1,6) macrocyclic glucan from Bradyrhizobium japonicum USDA 110. Biochim Biophys Acta. 1992 Jun 12;1116(3):215–225. doi: 10.1016/0304-4165(92)90014-l. [DOI] [PubMed] [Google Scholar]
- Stanfield S. W., Ielpi L., O'Brochta D., Helinski D. R., Ditta G. S. The ndvA gene product of Rhizobium meliloti is required for beta-(1----2)glucan production and has homology to the ATP-binding export protein HlyB. J Bacteriol. 1988 Aug;170(8):3523–3530. doi: 10.1128/jb.170.8.3523-3530.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Streeter J. G. Accumulation of alpha,alpha-trehalose by Rhizobium bacteria and bacteroids. J Bacteriol. 1985 Oct;164(1):78–84. doi: 10.1128/jb.164.1.78-84.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Carbohydrate, organic Acid, and amino Acid composition of bacteroids and cytosol from soybean nodules. Plant Physiol. 1987 Nov;85(3):768–773. doi: 10.1104/pp.85.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully R. E., Keister D. L., Gross K. C. Fractionation of the beta-Linked Glucans of Bradyrhizobium japonicum and Their Response to Osmotic Potential. Appl Environ Microbiol. 1990 Jun;56(6):1518–1522. doi: 10.1128/aem.56.6.1518-1522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh D. T., Reed R. H., Herbert R. A. The role of trehalose in the osmoadaptation of Escherichia coli NCIB 9484: interaction of trehalose, K+ and glutamate during osmoadaptation in continuous culture. J Gen Microbiol. 1991 Apr;137(4):745–750. doi: 10.1099/00221287-137-4-745. [DOI] [PubMed] [Google Scholar]
- Zevenhuizen L. P. Levels of trehalose and glycogen in Arthrobacter globiformis under conditions of nutrient starvation and osmotic stress. Antonie Van Leeuwenhoek. 1992 Jan;61(1):61–68. doi: 10.1007/BF00572124. [DOI] [PubMed] [Google Scholar]
- Zorreguieta A., Cavaignac S., Geremia R. A., Ugalde R. A. Osmotic regulation of beta(1-2) glucan synthesis in members of the family Rhizobiaceae. J Bacteriol. 1990 Aug;172(8):4701–4704. doi: 10.1128/jb.172.8.4701-4704.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iannino N. I., Ugalde R. A. Biosynthesis of cyclic beta-(1-3),beta-(1-6) glucan in Bradyrhizobium spp. Arch Microbiol. 1993;159(1):30–38. doi: 10.1007/BF00244260. [DOI] [PubMed] [Google Scholar]



