Abstract
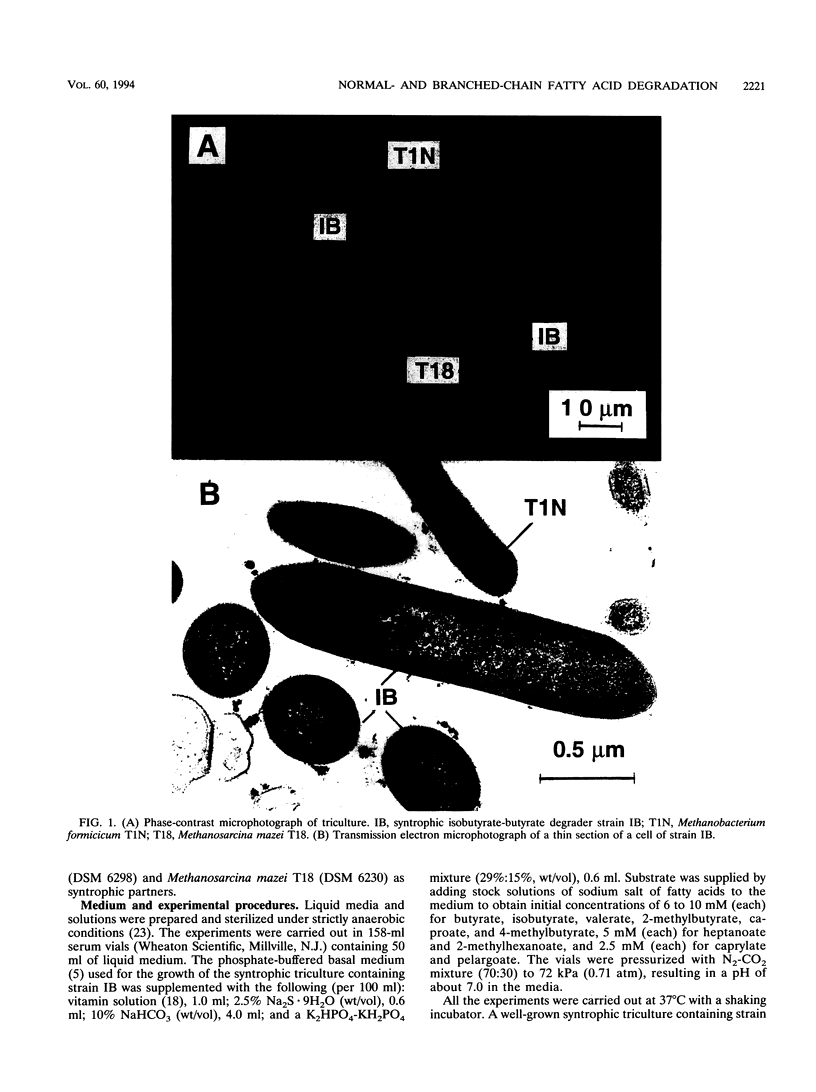
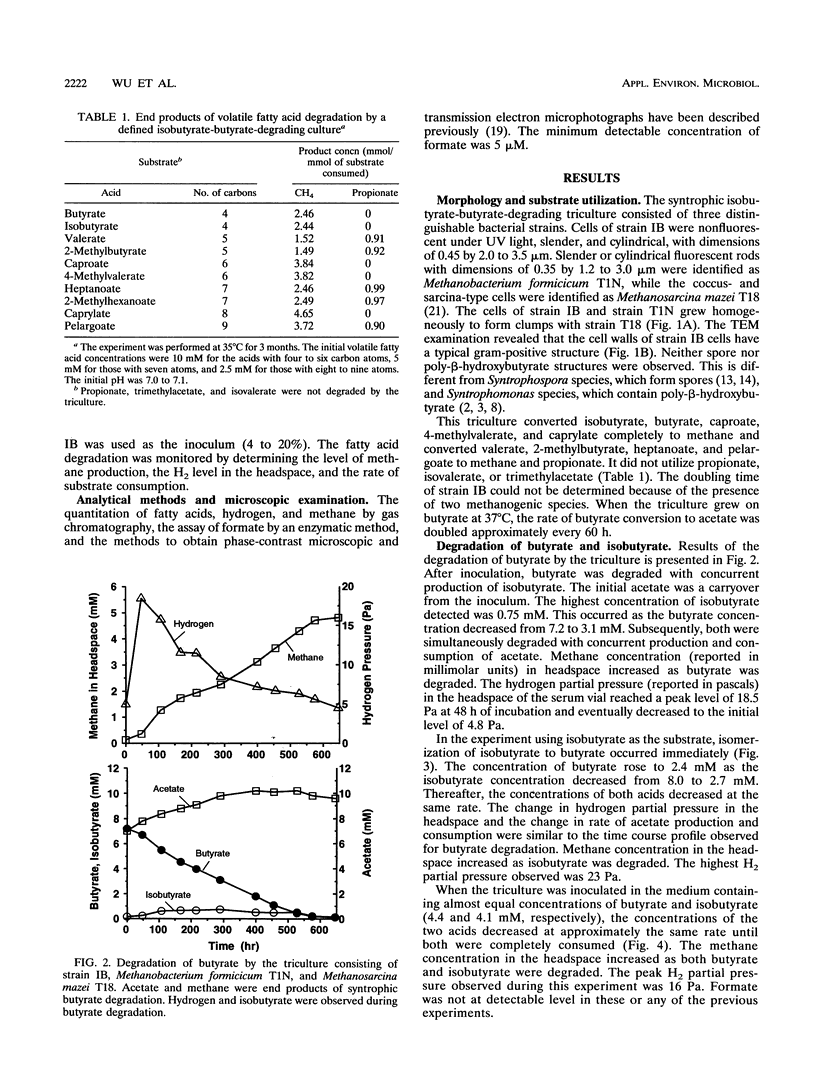
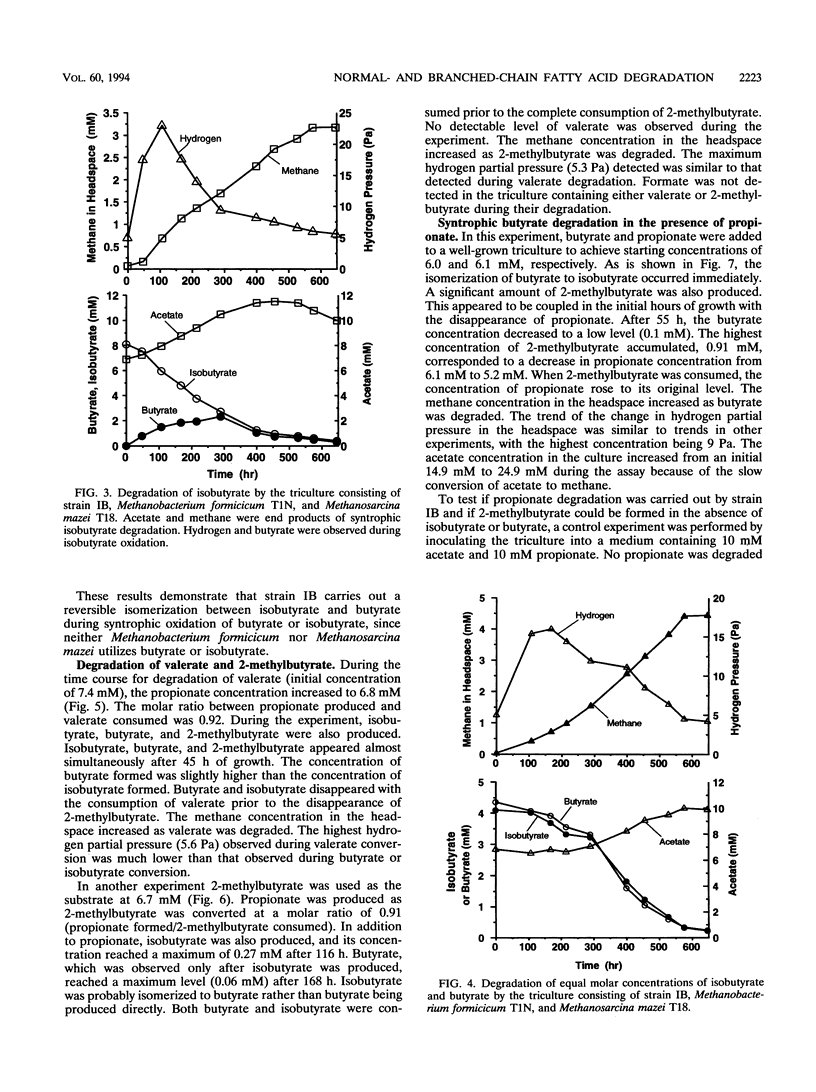
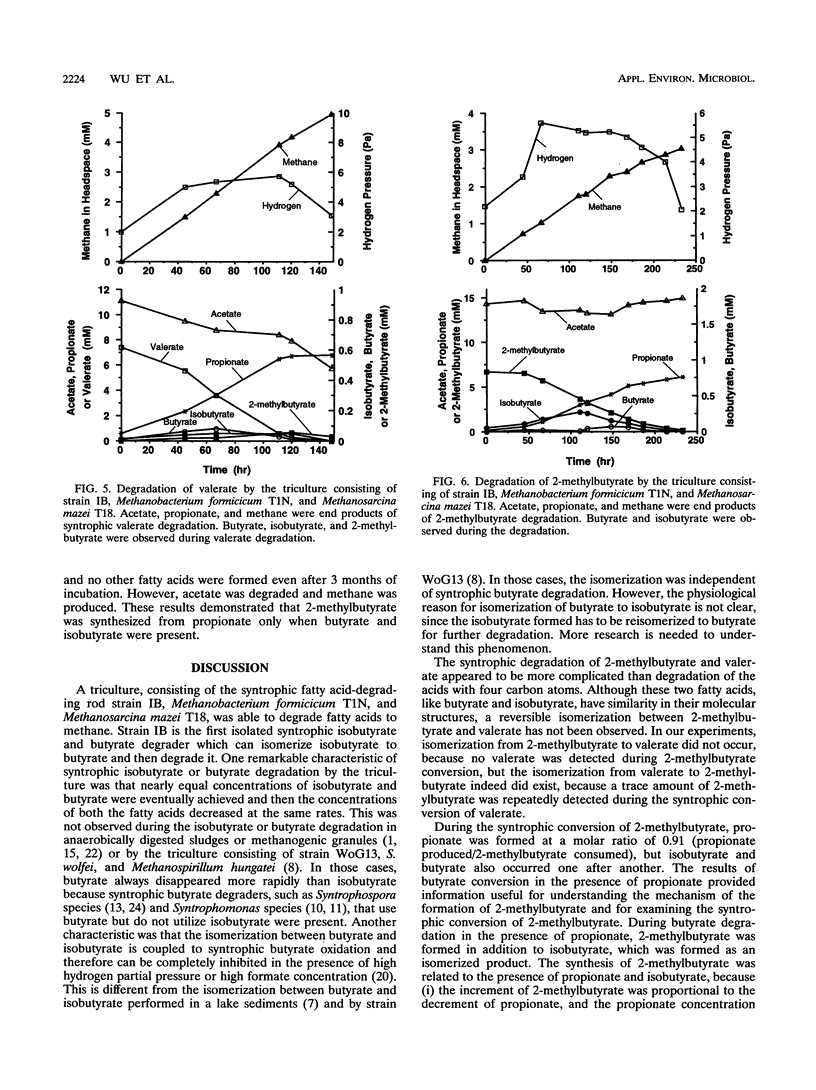
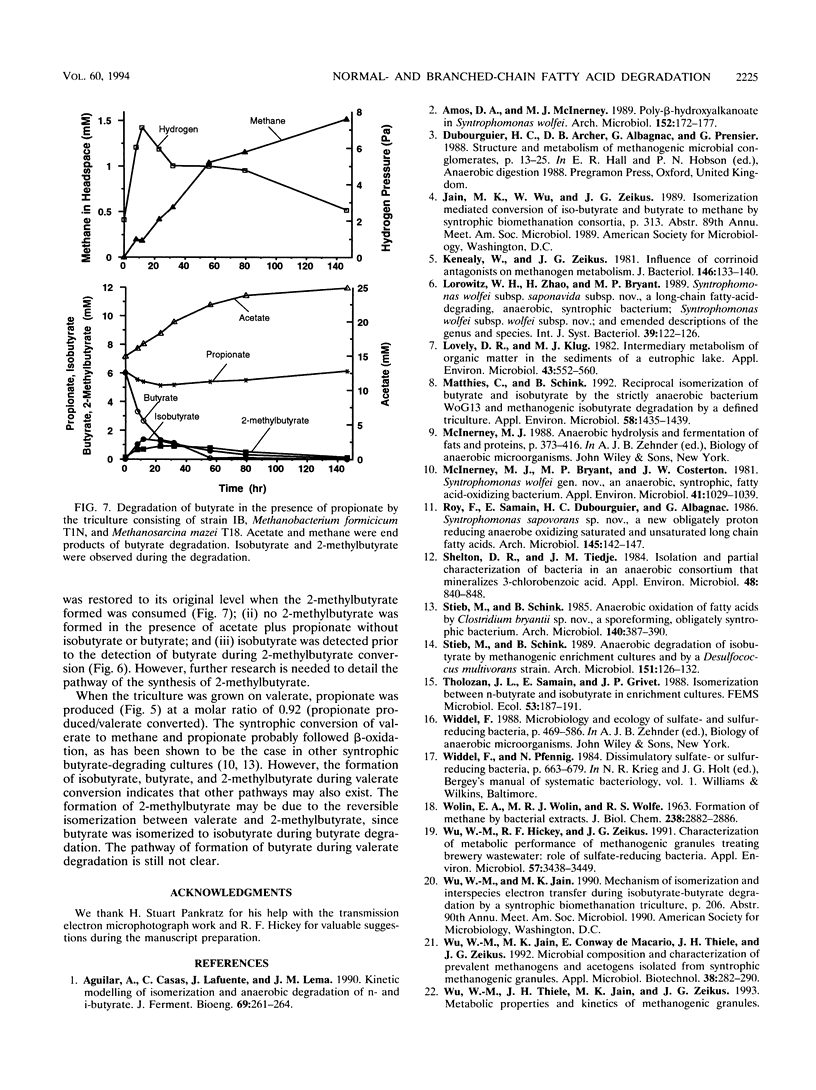
Syntrophic degradation of normal- and branched-chain fatty acids with 4 to 9 carbons was investigated with a mesophilic syntrophic isobutyrate-butyrate-degrading triculture consisting of the non-spore-forming, syntrophic, fatty acid-degrading, gram-positive rod-shaped strain IB, Methanobacterium formicicum T1N, and Methanosarcina mazei T18. This triculture converted butyrate and isobutyrate to methane and converted valerate and 2-methylbutyrate to propionate and methane. This triculture also degraded caproate, 4-methylvalerate, heptanoate, 2-methylhexanoate, caprylate, and pelargoate. During the syntrophic conversion of isobutyrate and butyrate, a reversible isomerization between butyrate and isobutyrate occurred; isobutyrate and butyrate were isomerized to the other isomeric form to reach nearly equal concentrations and then their concentrations decreased at the same rates. Butyrate was an intermediate of syntrophic isobutyrate degradation. When butyrate was degraded in the presence of propionate, 2-methylbutyrate was synthesized from propionate and isobutyrate formed from butyrate. During the syntrophic degradation of valerate, isobutyrate, butyrate, and 2-methylbutyrate were formed and then degraded. During syntrophic degradation of 2-methylbutyrate, isobutyrate and butyrate were formed and then degraded.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kenealy W., Zeikus J. G. Influence of corrinoid antagonists on methanogen metabolism. J Bacteriol. 1981 Apr;146(1):133–140. doi: 10.1128/jb.146.1.133-140.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies C., Schink B. Reciprocal isomerization of butyrate and isobutyrate by the strictly anaerobic bacterium strain WoG13 and methanogenic isobutyrate degradation by a defined triculture. Appl Environ Microbiol. 1992 May;58(5):1435–1439. doi: 10.1128/aem.58.5.1435-1439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P., Hespell R. B., Costerton J. W. Syntrophomonas wolfei gen. nov. sp. nov., an Anaerobic, Syntrophic, Fatty Acid-Oxidizing Bacterium. Appl Environ Microbiol. 1981 Apr;41(4):1029–1039. doi: 10.1128/aem.41.4.1029-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Wu W. M., Hickey R. F., Zeikus J. G. Characterization of metabolic performance of methanogenic granules treating brewery wastewater: role of sulfate-reducing bacteria. Appl Environ Microbiol. 1991 Dec;57(12):3438–3449. doi: 10.1128/aem.57.12.3438-3449.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. X., Yang D. C., Woese C. R., Bryant M. P. Assignment of Clostridium bryantii to Syntrophospora bryantii gen. nov., comb. nov. on the basis of a 16S rRNA sequence analysis of its crotonate-grown pure culture. Int J Syst Bacteriol. 1990 Jan;40(1):40–44. doi: 10.1099/00207713-40-1-40. [DOI] [PubMed] [Google Scholar]
- Zinder S. H., Cardwell S. C., Anguish T., Lee M., Koch M. Methanogenesis in a Thermophilic (58 degrees C) Anaerobic Digestor: Methanothrix sp. as an Important Aceticlastic Methanogen. Appl Environ Microbiol. 1984 Apr;47(4):796–807. doi: 10.1128/aem.47.4.796-807.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]