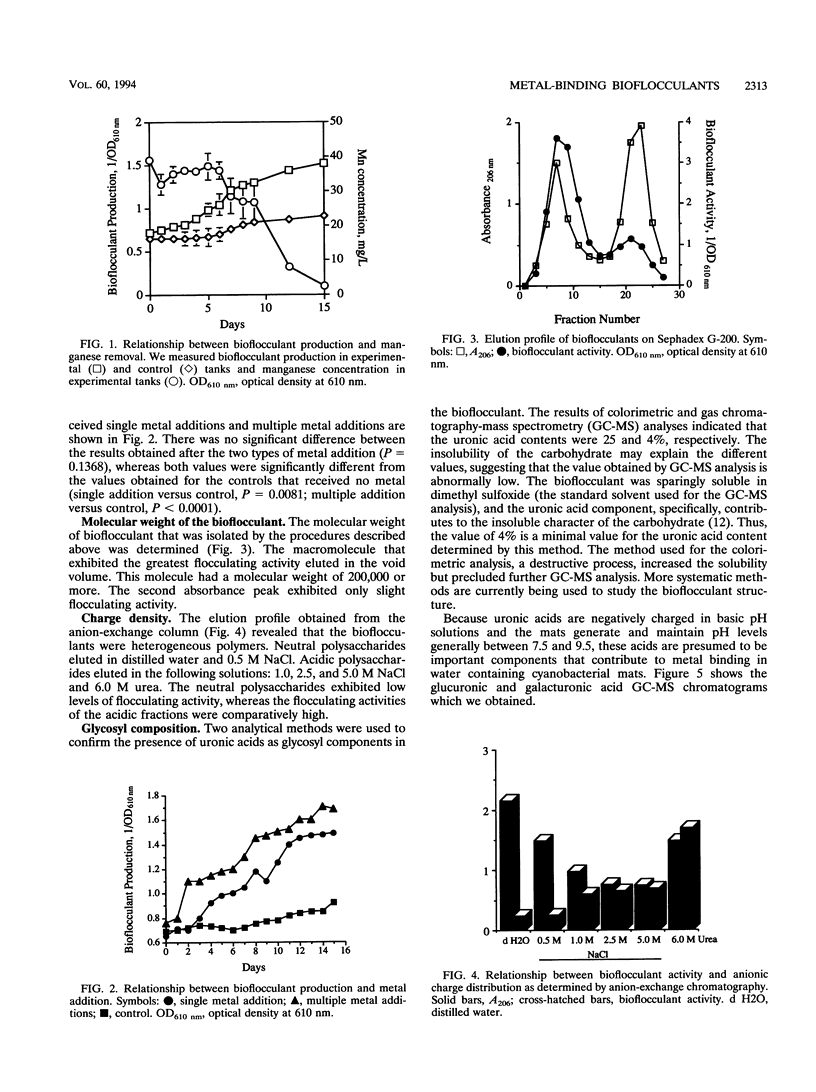
Abstract
Mixed-species microbial mats that were dominated by the cyanobacterium Oscillatoria sp. and contained heterotrophic and purple autotrophic bacteria were constructed for specific bioremediation applications. When the mats were challenged with metals, production and secretion of metal-binding extracellular polysaccharide bioflocculants were observed. The concentration of these negatively charged polysaccharides was correlated with the removal of manganese from the water column beneath a surface microbial mat. Bioflocculants from an Oscillatoria sp. that was isolated from the mat were collected and concentrated for characterization. A chromatographic analysis revealed a heterogeneous population of polysaccharides with respect to charge density and molecular size. The subpopulation of polysaccharides which exhibited the highest level of flocculating activity was polyanionic and had a molecular weight of more than 200,000. A glycosyl analysis of the bioflocculants revealed the presence of galacturonic acid (2.2%) and glucuronic acid (1.86%). The presence of these components, which were negatively charged at the pH levels generated by the mats during photosynthesis (pH > 7.5), may account for the metal-binding properties of the mats.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Or Y., Shilo M. Characterization of Macromolecular Flocculants Produced by Phormidium sp. Strain J-1 and by Anabaenopsis circularis PCC 6720. Appl Environ Microbiol. 1987 Sep;53(9):2226–2230. doi: 10.1128/aem.53.9.2226-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. H., Mitchell R. The role of polymers in microbial aggregation. Annu Rev Microbiol. 1973;27:27–50. doi: 10.1146/annurev.mi.27.100173.000331. [DOI] [PubMed] [Google Scholar]
- Kaplan D., Christiaen D., Arad S. M. Chelating Properties of Extracellular Polysaccharides from Chlorella spp. Appl Environ Microbiol. 1987 Dec;53(12):2953–2956. doi: 10.1128/aem.53.12.2953-2956.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnagel E. A., McNeil M., Albersheim P., Dell A. Host-Pathogen Interactions : XXII. A Galacturonic Acid Oligosaccharide from Plant Cell Walls Elicits Phytoalexins. Plant Physiol. 1983 Apr;71(4):916–926. doi: 10.1104/pp.71.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. A., Buchanan-Smith J. G. A colorimetric method for the quantitation of uronic acids and a specific assay for galacturonic acid. Anal Biochem. 1992 Feb 14;201(1):190–196. doi: 10.1016/0003-2697(92)90194-c. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]


