Abstract
The efficacy of copper and silver ions, in combination with low levels of free chlorine (FC), was evaluated for the disinfection of hepatitis A virus (HAV), human rotavirus (HRV), human adenovirus, and poliovirus (PV) in water. HAV and HRV showed little inactivation in all conditions. PV showed more than a 4 log10 titer reduction in the presence of copper and silver combined with 0.5 mg of FC per liter or in the presence of 1 mg of FC per liter alone. Human adenovirus persisted longer than PV with the same treatments, although it persisted significantly less than HRV or HAV. The addition of 700 micrograms of copper and 70 micrograms of silver per liter did not enhance the inactivation rates after the exposure to 0.5 or 0.2 mg of FC per liter, although on some occasions it produced a level of inactivation similar to that induced by a higher dose of FC alone. Virus aggregates were observed in the presence of copper and silver ions, although not in the presence of FC alone. Our data indicate that the use of copper and silver ions in water systems may not provide a reliable alternative to high levels of FC for the disinfection of viral pathogens. Gene probe-based procedures were not adequate to monitor the presence of infectious HAV after disinfection. PV does not appear to be an adequate model viral strain to be used in disinfection studies. Bacteroides fragilis bacteriophages were consistently more resistant to disinfection than PV, suggesting that they would be more suitable indicators, although they survived significantly less than HAV or HRV.
Full text
PDF
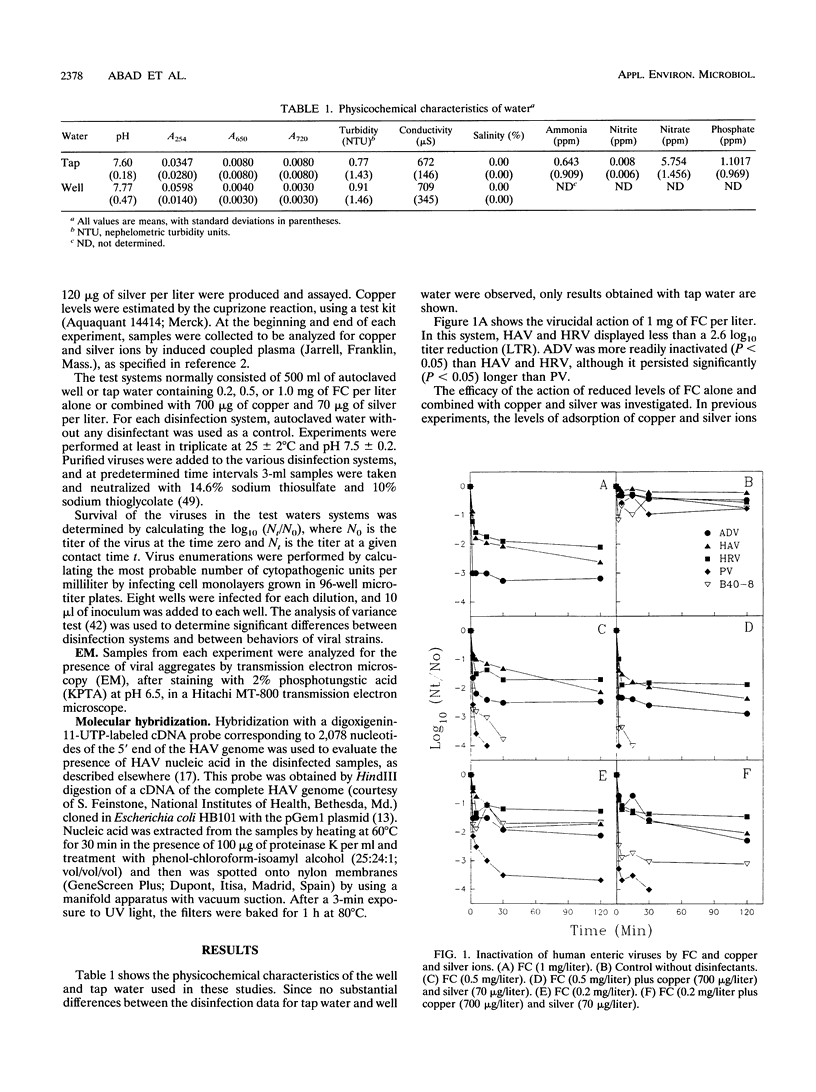



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron R. C., Murphy F. D., Greenberg H. B., Davis C. E., Bregman D. J., Gary G. W., Hughes J. M., Schonberger L. B. Norwalk gastrointestinal illness: an outbreak associated with swimming in a recreational lake and secondary person-to-person transmission. Am J Epidemiol. 1982 Feb;115(2):163–172. doi: 10.1093/oxfordjournals.aje.a113287. [DOI] [PubMed] [Google Scholar]
- Berman D., Hoff J. C. Inactivation of simian rotavirus SA11 by chlorine, chlorine dioxide, and monochloramine. Appl Environ Microbiol. 1984 Aug;48(2):317–323. doi: 10.1128/aem.48.2.317-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Ticehurst J. R., Purcell R. H., Buckler-White A., Baroudy B. M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987 Jan;61(1):50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromeans T., Sobsey M. D., Fields H. A. Development of a plaque assay for a cytopathic, rapidly replicating isolate of hepatitis A virus. J Med Virol. 1987 May;22(1):45–56. doi: 10.1002/jmv.1890220107. [DOI] [PubMed] [Google Scholar]
- D'Alessio D., Minor T. E., Allen C. I., Tsiatis A. A., Nelson D. B. A study of the proportions of swimmers among well controls and children with enterovirus-like illness shedding or not shedding an enterovirus. Am J Epidemiol. 1981 May;113(5):533–541. doi: 10.1093/oxfordjournals.aje.a113129. [DOI] [PubMed] [Google Scholar]
- De Leon R., Matsui S. M., Baric R. S., Herrmann J. E., Blacklow N. R., Greenberg H. B., Sobsey M. D. Detection of Norwalk virus in stool specimens by reverse transcriptase-polymerase chain reaction and nonradioactive oligoprobes. J Clin Microbiol. 1992 Dec;30(12):3151–3157. doi: 10.1128/jcm.30.12.3151-3157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Aggregation of poliovirus and reovirus by dilution in water. Appl Environ Microbiol. 1977 Jan;33(1):159–167. doi: 10.1128/aem.33.1.159-167.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajardo R., Díez J. M., Jofre J., Bosch A. Adsorption-elution with negatively and positively-charged glass powder for the concentration of hepatitis A virus from water. J Virol Methods. 1991 Feb-Mar;31(2-3):345–351. doi: 10.1016/0166-0934(91)90172-v. [DOI] [PubMed] [Google Scholar]
- Havelaar A. H., van Olphen M., Drost Y. C. F-specific RNA bacteriophages are adequate model organisms for enteric viruses in fresh water. Appl Environ Microbiol. 1993 Sep;59(9):2956–2962. doi: 10.1128/aem.59.9.2956-2962.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley H. B., Morin D. P., Geraghty M. E., Tomkow J., Phillips C. A. Coxsackievirus B epidemic at a Boy's Summer Camp. Isolation of virus from swimming water. JAMA. 1973 Oct 1;226(1):33–36. [PubMed] [Google Scholar]
- Jiang X., Estes M. K., Metcalf T. G. Detection of hepatitis A virus by hybridization with single-stranded RNA probes. Appl Environ Microbiol. 1987 Oct;53(10):2487–2495. doi: 10.1128/aem.53.10.2487-2495.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecka H., Dubrou S., Prevot J., Marechal J., López-Pila J. M. Detection of naturally occurring enteroviruses in waters by reverse transcription, polymerase chain reaction, and hybridization. Appl Environ Microbiol. 1993 Apr;59(4):1213–1219. doi: 10.1128/aem.59.4.1213-1219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeen L. K., Yahya M. T., Gerba C. P. Efficacy of copper and silver ions and reduced levels of free chlorine in inactivation of Legionella pneumophila. Appl Environ Microbiol. 1989 Dec;55(12):3045–3050. doi: 10.1128/aem.55.12.3045-3050.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985 Oct 24;313(17):1059–1067. doi: 10.1056/NEJM198510243131706. [DOI] [PubMed] [Google Scholar]
- Martone W. J., Hierholzer J. C., Keenlyside R. A., Fraser D. W., D'Angelo L. J., Winkler W. G. An outbreak of adenovirus type 3 disease at a private recreation center swimming pool. Am J Epidemiol. 1980 Feb;111(2):229–237. doi: 10.1093/oxfordjournals.aje.a112890. [DOI] [PubMed] [Google Scholar]
- Peterson D. A., Hurley T. R., Hoff J. C., Wolfe L. G. Effect of chlorine treatment on infectivity of hepatitis A virus. Appl Environ Microbiol. 1983 Jan;45(1):223–227. doi: 10.1128/aem.45.1.223-227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni A., Chevion M., Czapski G. Roles of copper and O(2) in the radiation-induced inactivation of T7 bacteriophage. Radiat Res. 1984 Sep;99(3):562–572. [PubMed] [Google Scholar]
- Sattar S. A., Raphael R. A., Springthorpe V. S. Rotavirus survival in conventionally treated drinking water. Can J Microbiol. 1984 May;30(5):653–656. doi: 10.1139/m84-097. [DOI] [PubMed] [Google Scholar]
- Tartera C., Jofre J. Bacteriophages active against Bacteroides fragilis in sewage-polluted waters. Appl Environ Microbiol. 1987 Jul;53(7):1632–1637. doi: 10.1128/aem.53.7.1632-1637.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman R. B., Gerba C. P. Molecular mechanisms of viral inactivation by water disinfectants. Adv Appl Microbiol. 1988;33:75–105. doi: 10.1016/s0065-2164(08)70205-3. [DOI] [PubMed] [Google Scholar]
- Vaughn J. M., Chen Y. S., Thomas M. Z. Inactivation of human and simian rotaviruses by chlorine. Appl Environ Microbiol. 1986 Feb;51(2):391–394. doi: 10.1128/aem.51.2.391-394.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahya M. T., Landeen L. K., Messina M. C., Kutz S. M., Schulze R., Gerba C. P. Disinfection of bacteria in water systems by using electrolytically generated copper:silver and reduced levels of free chlorine. Can J Microbiol. 1990 Feb;36(2):109–116. doi: 10.1139/m90-020. [DOI] [PubMed] [Google Scholar]
- Yahya M. T., Straub T. M., Gerba C. P. Inactivation of coliphage MS-2 and poliovirus by copper, silver, and chlorine. Can J Microbiol. 1992 May;38(5):430–435. doi: 10.1139/m92-072. [DOI] [PubMed] [Google Scholar]
- Young D. C., Sharp D. G. Poliovirus aggregates and their survival in water. Appl Environ Microbiol. 1977 Jan;33(1):168–177. doi: 10.1128/aem.33.1.168-177.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]